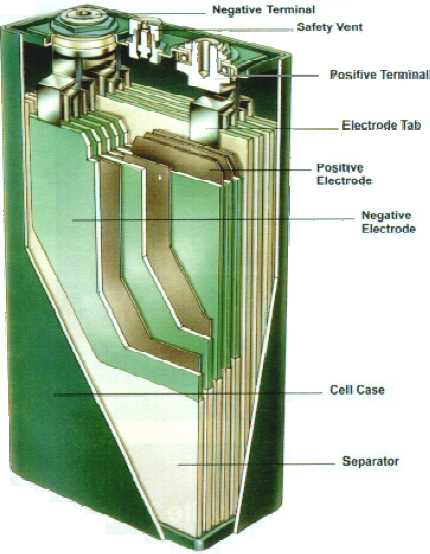
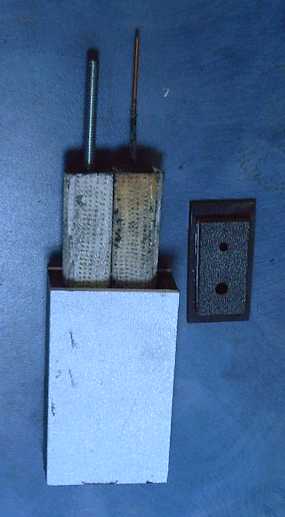
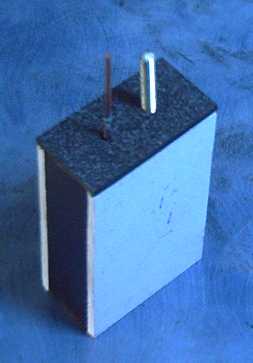
Handy Battery Sticks, 12 volt, 6 volt

30 D cells worth, 12 v, 30 AH, runs my car.
NiMH is a perfect drop-in
replacement for lead-acid in cars.


Later I made "quintos battery sticks", 12 volters to fit properly where
lead-acids used to go.
Still later I made 70 amp-hour, 12 volt "Super Battery Sticks" for
electric cars.