
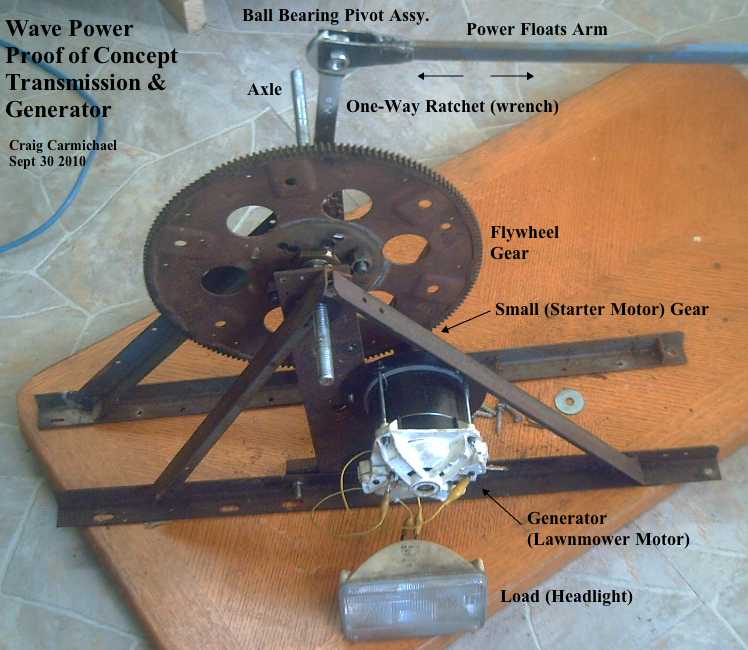
"Production" EH wheel motor mechanics layout.
1-1/2" cast steel pipe couplings machined into bearing hubs and welded to '6129' disk brake rotors,
on independently rotating (for torque converter output rotor) dexter trailer axle.












| Table I. Desirable properties of carbon and |
| graphite for electrochemical applications |
| • good electrical conductivity |
| • acceptable corrosion resistance |
| • availability in high purity |
| • low cost |
| • high thermal conductivity |
| • dimensional and mechanical stability |
| • light in weight and ease of handling |
| • availability in a variety of physical structures |
| • ease of fabrication into composite structures |



