
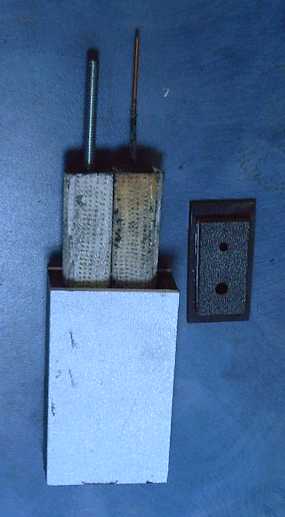
 Once I had the overvoltage problem figured out, manganese
for negatrodes at long last worked fine.
Once I had the overvoltage problem figured out, manganese
for negatrodes at long last worked fine. 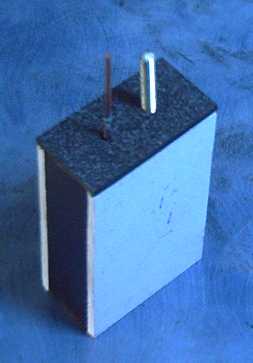 After four
years of mixed results at best, a battery with
all the features above worked well the day before my battery making
talk at Ideawave 2012. A Times-Colonist newspaper reporter interviewed
me, and I wrote him a brief description of the battery, leaving out
most of the ad nauseum technical details I always so scrupulously put
in. It probably bears reprinting here (re-edited):
After four
years of mixed results at best, a battery with
all the features above worked well the day before my battery making
talk at Ideawave 2012. A Times-Colonist newspaper reporter interviewed
me, and I wrote him a brief description of the battery, leaving out
most of the ad nauseum technical details I always so scrupulously put
in. It probably bears reprinting here (re-edited):




 * Take a piece of fat
polyolefin heatshrink tubing [electronics parts
store] and put lots of tiny
holes in it (sewing machine with no thread)
* Take a piece of fat
polyolefin heatshrink tubing [electronics parts
store] and put lots of tiny
holes in it (sewing machine with no thread)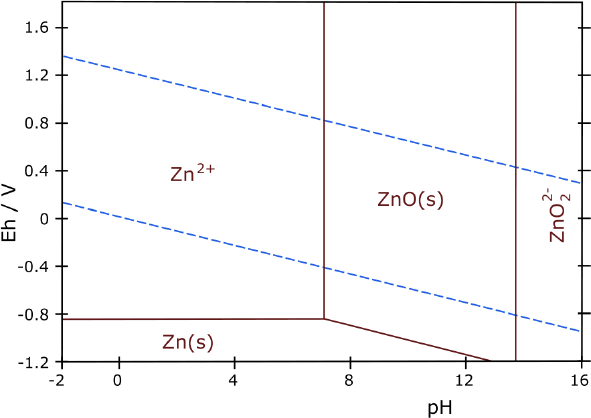 Zinc should
work without overvoltage raising... but better with it than without.
First I crunched up some stibnite with a hammer on an anvil to make it
as fine as possible, as I had noticed even the 'fine' powder did a lot
of crunching when ground with mortar and pestle - it's too coarse.
Veegum for glue. About
6 grams stuffed into the 'pouch', theoretically about 3-1/2 amp-hours
worth. The nickel electrodes should be of similar capacity.
Zinc should
work without overvoltage raising... but better with it than without.
First I crunched up some stibnite with a hammer on an anvil to make it
as fine as possible, as I had noticed even the 'fine' powder did a lot
of crunching when ground with mortar and pestle - it's too coarse.
Veegum for glue. About
6 grams stuffed into the 'pouch', theoretically about 3-1/2 amp-hours
worth. The nickel electrodes should be of similar capacity.

 The remaining
question then was whether it would be
practical to punch the multitude of tiny holes - preferably in the flat
sheet before forming it into a tube - assuming they wouldn't close up
when heated. If that could be done, it looked like I could soon have
real, working electrodes... followed by real, working batteries. Better
chemistry was merely a bonus!
The remaining
question then was whether it would be
practical to punch the multitude of tiny holes - preferably in the flat
sheet before forming it into a tube - assuming they wouldn't close up
when heated. If that could be done, it looked like I could soon have
real, working electrodes... followed by real, working batteries. Better
chemistry was merely a bonus! After thinking about a few ideas, I started to realize that 1/2" square
is probably just too fat. It's 6mm from the wire to the corners, where
other alkaline electrodes are 1mm or less from any point to a current
collector. 1/4" square electrodes would have four electrodes where one
1/2" one is. And they would be only 3mm to the corners (2mm with a fat
center conductor), and there'd be 4 wires in place of one. That's a
pretty small size to fill by hand, but undoubtedly more in line with
the electrochemical requirements. The big challenge then is to find
ways and means to make it very fast and easy to make each individual
small electrode. (How do those cigarette roller/stuffer things work?)
After thinking about a few ideas, I started to realize that 1/2" square
is probably just too fat. It's 6mm from the wire to the corners, where
other alkaline electrodes are 1mm or less from any point to a current
collector. 1/4" square electrodes would have four electrodes where one
1/2" one is. And they would be only 3mm to the corners (2mm with a fat
center conductor), and there'd be 4 wires in place of one. That's a
pretty small size to fill by hand, but undoubtedly more in line with
the electrochemical requirements. The big challenge then is to find
ways and means to make it very fast and easy to make each individual
small electrode. (How do those cigarette roller/stuffer things work?)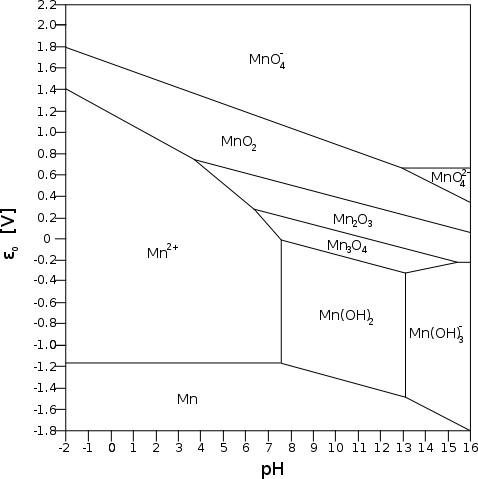 Referring to the Mn Pourbaix diagram, it appears that the manganate ion
will only form at pH 13 or above, but at about +.65 volts, which is 30%
higher energy than the .5 volts of the NiOOH reaction on top of the
higher amp-hours, and probably higher maximum amps capacity owing to
lower internal resistance with the manganate spinel structure.
Referring to the Mn Pourbaix diagram, it appears that the manganate ion
will only form at pH 13 or above, but at about +.65 volts, which is 30%
higher energy than the .5 volts of the NiOOH reaction on top of the
higher amp-hours, and probably higher maximum amps capacity owing to
lower internal resistance with the manganate spinel structure.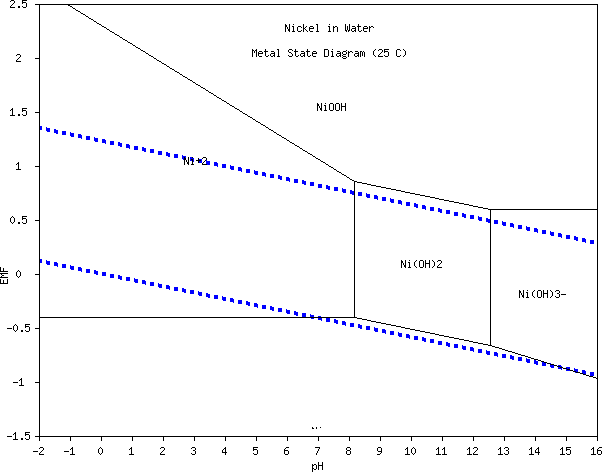 On
consideration, this or something akin seems more
likely. The
main differences then would be the high conductivity of nickel
manganate
compared to nickel hydroxide, allowing higher current flow, and that
nickel manganate and the charged coumpounds are (seem to be) insoluble
at neutral pH where the reaction voltage is around +.95, rather than
the usual +.5 in alkali.
On
consideration, this or something akin seems more
likely. The
main differences then would be the high conductivity of nickel
manganate
compared to nickel hydroxide, allowing higher current flow, and that
nickel manganate and the charged coumpounds are (seem to be) insoluble
at neutral pH where the reaction voltage is around +.95, rather than
the usual +.5 in alkali.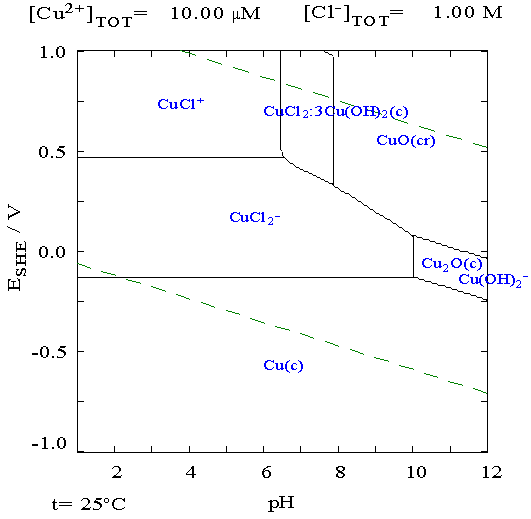 It occurred to me
that altho I had no 'standard
hydrogen electrode' with which to determine individual electrode
voltages, I could stick a copper wire into the liquid. With the pH and
a copper Pourbaix diagram, I could find the electrode voltage of copper
metal, and subtract that from a measured voltage to get the voltage of
individual electrodes. It appeared that in chloride solution (there
were four separate Pourbaix diagrams for copper in different solutions)
it should be -.13 volts from acidic pH up to about 10, but the readings
didn't jibe with approximately known figures. Maybe I should try zinc.
It occurred to me
that altho I had no 'standard
hydrogen electrode' with which to determine individual electrode
voltages, I could stick a copper wire into the liquid. With the pH and
a copper Pourbaix diagram, I could find the electrode voltage of copper
metal, and subtract that from a measured voltage to get the voltage of
individual electrodes. It appeared that in chloride solution (there
were four separate Pourbaix diagrams for copper in different solutions)
it should be -.13 volts from acidic pH up to about 10, but the readings
didn't jibe with approximately known figures. Maybe I should try zinc.