Turquoise
Energy Ltd. News #50
Victoria BC
Copyright 2012 Craig Carmichael - April 4th, 2012
http://www.TurquoiseEnergy.com
= http://www.ElectricHubcap.com
= http://www.ElectricWeel.com
Month In Brief (Summaries)
* Ni-Mn alkaline batteries work, and may be made in China by
ChangHong battery company
* Editorial: Social Sustainability
Feature: Magnetic Field Spacecraft Drive - The
future of space travel? and Magnetic Field Motor?
Magnetic Impulse Torque Converter Project
* Slow going putting together motorbike converter, then wrong chain type
* Torque pulses are too weak - sigh!
* And now for something completely different: centrifugal clutches
NiMH
Battery
Project
* Better economy for electric mowers
* Battery sticks V3: custom rolled plastic tubes - thinner, lighter;
better fit in
tight places
Turquoise
Battery Project
* Graphite powder in negatrode causes slight self discharge? - ugh!
* Zincate Solution - zincate on aluminum: no self discharge
* Contacted ChangHong Battery Co. about having them make NiMn flooded
alkaline cells.
* Zincated aluminum grill spiral wrapped electrode: a new and simple
way to make an electrode?, with better current capacity.
No Project Reports on: Electric
Hubcap motor system, Weel motor, Sprint car conversion, Electric
outboard
from scratch, LED Lighting Project, DSSC solar cells, Pulsejet steel
plate cutter
Newsletters
Index/Highlights: http://www.TurquoiseEnergy.com/news/index.html
Construction Manuals and information:
-
Electric Hubcap Motor - Turquoise Motor
Controller - 36 Volt Electric
Fan-Heater
- Nanocrystalline glass to enhance Solar
Cell performance - Ersatz 'powder coating' home process for
protecting/painting metal
Products Catalog:
- Electric Hubcap Motor Kit
- Sodium Sulfate - Lead-Acid battery longevity/renewal
- NiMH Handy Battery Sticks, Dry Cells
- LED Light Fixtures
Motor Building
Workshops
...all at: http://www.TurquoiseEnergy.com/
(orders: e-mail craig@saers.com)
March in Brief
The month started with 2 weeks of income tax (complete
with
several omissions and errors),
interspersed with some battery experiments and thinking about an idea
for a magnetic field spacecraft propulsion system, and superconductors
to make huge magnetic fields for that. It ended with an
idea for a magnet motor powered by Earth's magnetic field, a torque
converter that still wouldn't move even a motorbike while approaching
the
third anniversary of the project, and me feeling a little overwhelmed
by all my unfinished projects and exciting ideas for new ones.
In the battery experiments, I finally eliminated the last
of the main nagging self discharge problems by using 'zincate solution'
(can
be
purchased or made) to zinc coat aluminum rods instead of using
galvanized nails - I guess the zinc coating on the nails must be impure
or have
gaps. (The hardware store shall know of my displeasure!)
So (at long last) I had 2 volt alkaline batteries that
stayed charged -
great, but the conductivity was pathetic. They'd put out a few
milliamps for hours at ever drooping voltages, but they wouldn't hit
anything like an amp. I tried rolling up an electrode in zincated
aluminum mesh, with a zincated aluminum wire sticking up. That would
put far more metal in close touch with the negatrode substance, and
thin sheet aluminum proved to be half the weight of the plastic. It
proved surprisingly feeble. Perhaps my negatrodes were just too fat.
They'd have to have 1/4 as much material or be made with another plan,
such as flat plate grills.
But it made a battery, and after a few days of charging, I
did the following load test. It can be seen from the slope that it
could have continued discharging for many more hours, at ever dropping
voltages.
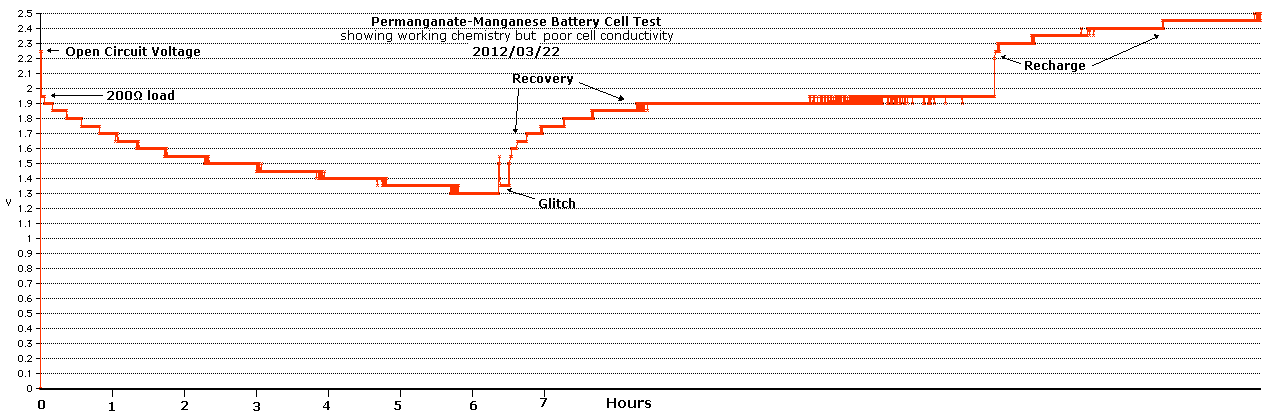
Tentatively, the square perf plastic pocket positrodes
seemed to hold their voltage somewhat better than the negatrodes. A
fatter center post wire seemed to help a lot; so did tapered posts, but
too many showed the turquoise color that said the copper wire inside
was oxidizing. One fat wire corroded off and the post was replaced for
the test
above.
I've decided to try making a tapered solid grafpoxy
equivalent to the fat carbon rods in 'D' dry cells, with a short copper
wire sticking out the top but not going in very far. I tried
sucking the grafpoxy into drinking straws with a big syringe, but it
seemed to leave hollow centers. Maybe some sort of sideways mold in
polyethylene plastic.
At last being pretty sure of the chemistry and essential
workings, on the 27th I sent an e-mail to ChangHong batteries in China.
Ian
Soutar, who has been dealing with them for some time, has been
suggesting this for some time, and sent a suitable introductory e-mail.
They
have a single assembly line (purchased from Varta in Germany) that
makes flooded alkaline pocket cell batteries, both Ni-Fe and Ni-Cd in
accordance with demand. It seemed to me they could almost as
easily plate their negative electrode pockets with zinc and make
manganese
electrodes, Ni-Mn, to get 60% higher energy and voltage using the same
assembly
line. That would make them very competitive for electric transport as
well as everything else.
ChangHong's initial perception that a new chemistry
would require making a wholly new type of battery, and their resistance
to considering that, showed the value of having suggested only this one
simple improvement to their existing product line. Offering more
improvements - safer electrolyte, better positive electrodes, new
electrode constructions - would only have confused the issue.
Ni-Mn in KOH cells should be about 1.9 volts nominal -
a 50-60% energy increase from the same cells off the same assembly line
with almost no more expense. Not only does ChangHong have one of only
three or four assembly lines in the world that could easily do this,
it's the battery supplier for the Chinese government and military, so
it seems unlikely it will be permitted to fall under the sway of
corrupt business interests that would be likely to shut it down or
curtail and ration its output.
I have already stated that if Canada didn't support and
commercialize my work, we'd soon be importing my inventions from other
lands. Why would my wave power designs not have gone forward when the
BC Ministry of Energy ocean energy official liked them so much in 2007,
to provide the power we need here on Vancouver Island? - we have the
best coastline in the world for it. I suspect granting me the few
thousand dollars I asked for a proof-of-concept unit would have been
the most important
thing he could have done in his whole tenure in that position, but he
had no authority to act even in a small way. Instead I left empty
handed, and now eight
billion or more dollars are to be spent on the Peace River Site C dam.
Similar wave power units have since been re-invented
independently in Denmark. The inventor got an award and government
funding - we may be importing the machines from there. Denmark can do
it; evidently BC and Canada won't or can't.
The fact
that there's really nowhere to go for support to create new things in
Canada makes it almost problematic. Except for tax credit
partial reimbursements for expenses already incurred, no one from the
Prime Minister on down who
has funding authority feels inventing the future has anything to do
with them. Avro Arrow, hydro dams and other notable Canadian
achievements aren't created just with verbal support or loaded IRAP 50%
funding. I suspect in China I'd be making a very good living for doing
the things I'm doing, and I've been told the government of Palestine
(of all places)
would dearly love to have me working there.
But ChangHong's assembly line won't go far to fill the
world's rapidly growing needs for more economical high energy
batteries. My chemistries
and designs for still better batteries are getting into more advanced
stages, and they are public. There's still room.
The magnetic field propulsion idea started with Jim
Harrington's study of
diamagnetics, materials repelled by any magnetic field, and
progressed to rare earth supermagnets that are much stronger and can
repel or attract, and then to
superconductors, which can create humungus magnetic fields, and
completely block fields trying to pass through them. The fields of the
craft propel it by attraction or repulsion of planetary
fields - Earth, the Sun, Jupiter, Ganymede...
An experiment (repeated in two locations for verification)
showed that supermagnets on a long thread hang slightly to the magnetic
south or to the north, depending which way around they're facing. It's
only around .001g with these magnets in Victoria BC, but motive force
is there.
Towards the
magnetic poles it should be considerably stronger.
That lead to thinking about making liquid nitrogen and
potential materials like glassy mixtures of lithium/beryllium/boron and
hydrides for higher
temperature superconductors, design of
superconducting magnet coils, etc. And it seems individual bismuth
needle-like crystals are superconducting up to 15ºC... what can be
done with that? Why did I have to think of this -
don't I
have enough to do? Others are downplaying the
potential, but I think they're glossing over the possibilities for
workable implementations, such as having (seemingly obvious) X Y Z axis
magnets for three axis stabilization, or rapid computer adjustments of
magnet orientation to maintain stability. It seems very exciting to me.
I'd
love to
start with three supermagnets (or one with the computerized stability)
and see if we could crash UVic's upcoming
Ecosat low Earth
orbiting satellite into the moon!
 At the very
end of the month, I thought of a more Earthly
application: It should be possible to get a magnet rotor to draw power
to rotate continuously from Earth's magnetic field if it was set up
right. But of the two forces - the one that wanted to twist the magnets
into magnetic alignment and the one that wanted to move them linearly,
the former was much the stronger, and that would need more than just
static magnets glued to a ring.
At the very
end of the month, I thought of a more Earthly
application: It should be possible to get a magnet rotor to draw power
to rotate continuously from Earth's magnetic field if it was set up
right. But of the two forces - the one that wanted to twist the magnets
into magnetic alignment and the one that wanted to move them linearly,
the former was much the stronger, and that would need more than just
static magnets glued to a ring.
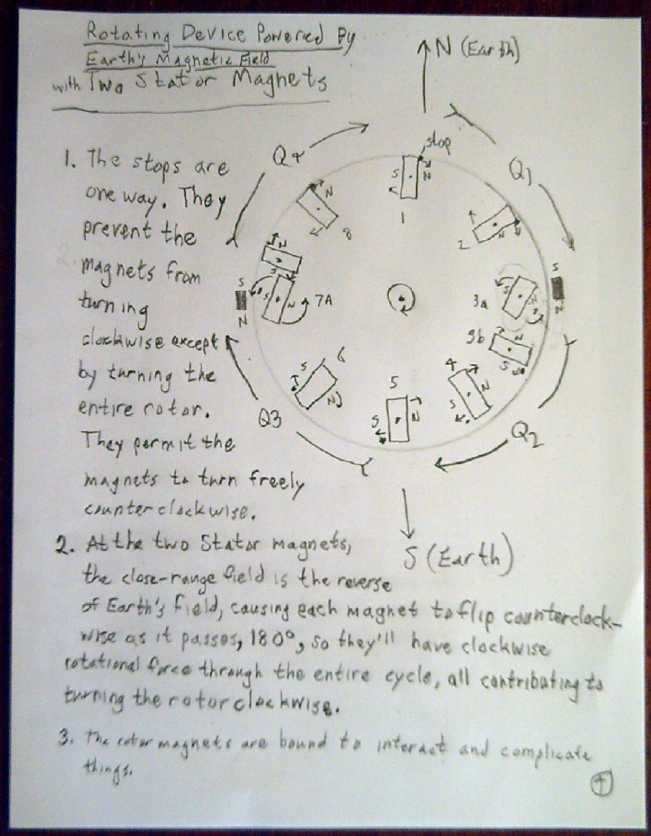
I quickly tried out a static rotor arrangement, but it
would barely even act as a compass and face north with three aligned
magnets, much less spin from the weaker linear force with nine magnets
appropriately oriented.
I figured out that the magnets on a rotor would have to
rotatable but blocked from turning (eg) clockwise, and would all have
to apply their clockwise "compass" twist to the whole rotor. Half way
between north and south, each magnet would have to be flipped 180º
counterclockwise as it passed small stator magnets that would overpower
the Earth's field only right at the two midpoints. The forces are small
and the friction would have to be overcome. Furthermore, the
interactions between the magnets might well cause problems... but
otherwise the theory seems good.
 As expected,
it wasn't until late in the month that I got
back to the torque converter, to make a magnetic impulse only
implementation for the motorbike. I really wanted to get that done and
test it.
But "the devil in the details" was on his best game. The work, which
seemed simple enough in principle, proceeded painfully slowly. I
'finished' it on the 29th and made a short demo video on the morning of
the 30th, but when I put it on the bike, I found changes were needed to
get it to work.
As expected,
it wasn't until late in the month that I got
back to the torque converter, to make a magnetic impulse only
implementation for the motorbike. I really wanted to get that done and
test it.
But "the devil in the details" was on his best game. The work, which
seemed simple enough in principle, proceeded painfully slowly. I
'finished' it on the 29th and made a short demo video on the morning of
the 30th, but when I put it on the bike, I found changes were needed to
get it to work.
On the 31st I got some parts and by the evening of April
1st I had it mounted with most of the adjustments done. On the 2nd it
was cludjed together enough to try it... and the Sun was out! The
pulses of torque were too
weak, even for just a motorbike. I could have improved on it with a
more magnets and copper, and maybe even got it to work. But it no
longer seemed terribly promising.
Partly because a highly suitable new centrifugal clutch
had been brought to my attention at the store, I decided to use it to
try an approach somewhat similar to the unfinished mechanical one prior
to the magnetic idea. This unit was almost bound to work because its
sprocket gear with only 10 teeth would give 6 to 1 reduction - probably
enough to run the bike even with no torque converter. The improvements
the clutch part made could be measured, and there were a couple of
things I could try out using it.
Editorial - Sustainability: Physical and Social/Political
Abraham Lincoln, IIRC, said that no foreign power could
take away America's freedoms. If it was to lose them, it would be
internal. In the last decade, the US congress has transferred many
of its own valuable rights and prerogatives to the presidency (while
they play computer card games and chat on Facebook during congressional
sessions).
But I'm agog
that they would pass a bill that suspends the centuries old right of
habeas corpus - no imprisonment without criminal charges, a trial and
conviction. Soon in the USA anyone who's inconvenient
to the government will be simply thrown in jail, even for life, and
"terrorist" profiles - with the definition and determination of
"terrorist" resting with the enforcement agencies - are even now being
drawn up against everyone who
has spoken out for change and progress in recent years.
This is a devastating blow to liberty. Freedom of speech is vanishing.
No one is safe. All the
terror apparatus of a totalitarian state is swiftly being set up, and
the USA now just needs an aspiring dictator to step in and use for his
own ends the
powers and organization that have been conveniently provided.
Short of nuclear annihilation, this indeed appears far
worse
for America than anything any foreign enemy could possibly have
inflicted on it.
Turquoise Energy has been all about helping to evolve
physically sustainable energy systems. An even higher cause is to
develop
socially sustainable systems. My proposed Department of Progress
and the principles outlined in Fundamental
Principles of Democratic
Government - towards utopian systems of governance could be
cogs in
that wheel of sociopolitical progress.
Today's superficially democratic and fundamentally
dysfunctional systems of government badly need to evolve, but those
who make it into power by 'virtue' of personal ambition saying "I can
impose my philosophy and things will be better" - which has been
gradually devolving to "I and my corporate friends can make piles of
money if
I attain political power" - have done so using the system as it is, so
when they get there, they think it's a great system - it worked for
them. Those who say "I can serve better to help create a peaceful,
sustainable
world", and original thinkers who might try to remedy deficiencies in
the system, are shut out of the decision making process and out of the
economic system by the very problems with that system. (And indeed
those who speak up now appear to be in peril.) Political
progress doesn't happen and seems impossible. We seem to have
have
come to an evolutionary dead end.
How did we get to this point? We can go back to the
beginnings of democracy. Those who set up the governing systems we have
today saw them as great improvements over what existed before - and
they were. But they couldn't be all-wise and foresee all the
consequences before the ideas were tried. The "devil in the details"
could only be discerned over long periods of time, and by then the
systems had become
traditions, "set in stone".
The "illiterate's X" voting system was a big improvement
over having no choices at all. But wherever there are more than two
choices, not being able to rank them in order of preference and have
the election results follow that ranking - so simple to do - brings
about
an unfair situation that polarizes the whole process and system.
Instead of being a representative cross section of the electorate where
bills are proposed, debated and enacted, legislatures become the site
of a struggle for dominance between a small number of political
factions who make their decisions behind closed partisan "party" doors
and impose them if they can dominate the legislature, which then simply
becomes a place where these partisan decisions are rubber stamped.
Nor was it foreseen that parliament would become the
executive seat of government as well as a legislature. The English
kings who called parliaments were the executive leaders. Of course, the
hereditary process of choosing the leader led to many unfit leaders,
some much too young, and those who had 'been there too long for any
good they might have done'. Parliament usurped the role in the English
civil war, reducing the monarchy to a figurehead position. At that
point, the executive and legislative branches of government had been
inadvertently combined, to the detriment of both functions, and this
dysfunctional system was passed on to many other lands.
After the industrial revolution, as industry and commerce
grew, they were never properly brought under the control of the not
entirely functional elected governments. In some lands in the early
20th century, dictatorships fostered and abetted by industry usurped
power. (This nearly happened in the USA in the 1930's - the well
developed plot was foiled when the hard line general the corporate
conspirators picked to be dictator instead blew the whistle to
congress.) It took a world war to reverse the process and start
restoring and extending democracy.
I see 1948 as a pivotal point. When after a long trial GM,
Standard
Oil, Phillips Petroleum and Firestone Tire were convicted of the
conspiracy by which they had shut down and ripped out America's entire
trolley/rail transportation system, city by city, in the 1920's, 1930's
and
1940's, the companies received token fines and no responsibility was
accorded to the individual conspirators.
If any person had gone to a
trolley yard
and set fire to a trolley car, he'd have gone to jail. Alfred Sloan (GM
president) and his cohorts burned them by the thousands and ripped up
the tracks for
selfish greed: in order to immediately sell diesel buses, petroleum and
tires, and in the longer term to force people to buy gasoline guzzling
cars. (They had already killed production of electric cars and
(specifically GM/Sloan in 1926) Constantinesco's mechanical torque
converter cars that used half
the fuel. FDR's secretary of state had accused multimillionaire Sloan
of being an unpatriotic lowlife a good 15 years previously. At GM
headquarters was a folder full of newspaper articles collected from
city after city telling of the horror, disgust and outrage of the
people when their trolley lines were ripped out.) They
committed socioeconomic treason: the utter destruction of
the whole
beloved electric transportation grid and infrastructure built up over
decades
by the hard earned dollars of the American taxpayer. And they did it
with impunity.
This travesty of justice sent a clear
message and precedent that western, or least
American, society would tolerate and overlook any crime, no matter how
heinous, no matter how ruinous, as long as it was done under corporate
auspices as a "business strategy". The die was then cast for ongoing
future outrages leading to economic dictatorship, and the gradual
deterioration and decline of western civilization, which I have been
watching with amazement happening over my lifetime, great scientific
and material progress in many areas notwithstanding.
I can't help but think that big companies would have a
wholly different attitude today if Sloan and his chief partners in
crime
had been hanged for their many crimes, which were cynically executed
with malice aforethought and ruinous far, far beyond murder.
So... where do we go from here?
The biggest evolutions of government weren't gradual, they
were sudden mutations. Without discussing earlier Greek and Roman
democratic forms, the
first English parliament was created by Simon de Montfort, who first
created a council of 15 nobles to impose decisions on his brother in
law, the irresolute king Henry III. Henry recovered ascendancy, then
was defeated in battle by de Montfort, who created a council of 9 and
ruled "in the name of the king", who was imprisoned. Wanting the
support of the middle
classes, de Montfort then called together knights from every shire and
burgesses from selected towns, who first met in 1265. Although the
parliamentary form was retained by Henry's son Edward I (who defeated
de Montfort) and gradually evolved from there, it was born
as a sudden mutation, an idea by one man.
The American constitution and
political system weren't conceived by a long drawn out process of
committees and consultations. They were drawn up by a small group of
people with some new ideas, and (I hear) some ideas from the Iroquois
constitution - I suspect in good part
it was formulated by inventor
and writer Benjamin
Franklin. (but I haven't checked this out.) This new mutation not only
created legislatures, but replaced
the hereditary monarch with an elected representative of the people and
a limited term of office, while restoring a balance of powers between
the executive and legislative branches. The only political part of the
process was for the states to vote to accept what had been offered.
Rule by monarchies, dictatorships, and lately communism
became socially and morally bankrupt and these have mostly given way to
more
inclusive systems. But today most established parliamentary democracies
and
American democracy are also pretty much socially and morally bankrupt,
uncontrolled economic tyranny having (so far) replaced direct political
tyranny.
We need to transcend the problems that have led to
the current impasse and repair the systemic causes. The now
disintegrating
systems that nurtured the problems can't repair them or themselves from
within. The
world is just about ready for
another sudden mutation to more functional
and sustainable democratic systems and forms that prevent rogue
elements from
seizing economic control, to usher in a utopian future. When the time
is ripe, it will appear. When will the time be ripe?
Lucifer and his gang are gone and only the last
manifestations of their selfish way of thinking - promulgating
unreasoned fear leading to extremes of stagnation, hoarding and war -
remain to be eliminated -- and the individuals caught up in these
thought patterns forgiven. The angels say
"Fear not!" They are on our
side and there
is a cosmic plan for this next sudden mutation, for the restoration
and progress of our sphere.
But it
always comes down to each one of us. Change in the outer world starts
with change from within. As the many begin to pray for enlightenment,
progress
and courage, then pause a few minutes to reflect for an answer,
enlightened and
courageous spirit led individuals will start to create an enlightened
and
progressing
culture. Then the time will be ripe. In a new and improved
translation of Jesus' Aramaic words: "Blessed are the moderate, for
they shall inherit the Earth." ...but I'm sure I'm preaching to the
converted!
Magnetic Field Spacecraft Propulsion - Magnetic Field
Motor
A team at the University of Victoria was planning to
launch a small satellite with a camera on board, "Ecosat", into low
Earth orbit. The infra-red camera
science
payload plan proved unworkable and was abandoned by the proponent.
Suddenly, there was a satellite team with an empty satellite shell, and
a launch set up, but no reason to launch it. The word went out locally
that anyone who had some science project that could best be done in
space could have a satellite to put it in and a team to operate it. Jim
Harrington (who once made an energetic particle telescope (EPAC) for
the Ulysses spacecraft that studied the sun from polar orbit), proposed
to do an experiment making use of diamagnetic materials and doped
fluorites to propel or orient a spacecraft.
Diamagnetic materials are repelled by both north and south
magnetic fields. You can levitate a flat piece of pyrolytic graphite
just above supermagnets.
Supermagnets themselves can be levitated by flat pieces of bismuth, and
Harrington discovered that certain fluorite crystals can
be strongly
diamagnetic. He
proposed that they could be repelled by magnets on board a spacecraft
and the spacecraft would accelerate. They could also be used to
orient the spacecraft. A frictionless magnetic orientation system
sounded plausible, but I said the magnet would be repelled equally the
other way, and to think the arrangement would propel a spacecraft
sounded suspiciously like perpetual motion.
He replied that space is full of magnetic fields. This is
true, and suddenly it didn't sound so silly. A material that
could selectively be repelled or be neutral to a planet's magnetic
field depending on orientation, like pyrolytic graphite, would allow
both acceleration and navigation. The equal and opposite reaction of
the acceleration would act against a whole planet, or the sun, through
its magnetic field. Here, then, was the holy grail: weightless rocket
fuel - the future of space travel!
In fact, the more one thinks about it, the more practical
it seems. At least the Earth, the Sun, Jupiter, Ganymede and Saturn
have magnetic fields. They aren't absent even in interstellar space.
Harrington had the idea of sending a probe to Alpha Centauri, which
would gradually accelerate to near the speed of light and arrive in
years rather than centuries.
Closer to home, Earth's field turns magnets into
alignment with the magnetic poles.
Unnoticed, if the compass is closer to (eg) the north pole than the
south, it's actually being slightly pulled towards it. If you turn the
compass needle around, it's being pushed away. If it can orient
material on the ground where there's friction, it can certainly orient
it, and propel it, in space where there isn't.
The main things that have been used or proposed to propel
objects in space so far are rocket propulsion, ion drive, the solar
wind, and the weight of photons - light from the sun. The first two
work, but mass must be expended, limiting their operating time. Nothing
pushing mass out the back can continually propel a craft in space for
months or years as it flies towards a destination. Either the solar
wind or light pressure would
require sail areas measured in square kilometers of gossamer weight
material to give a spacecraft even discernable acceleration -
calculations show disappointing thrust. The forces
of Earth's magnetic field are surely orders of magnitude stronger - the
compass needle moves readily. A spacecraft could easily be oriented -
and also propelled - by the same force.
My first organized thoughts for implementation were to
have rotatable pieces of pyrolytic graphite at distance 'n' from the
center of mass on the X Y and Z axies. This would give the craft three
axis stabilization, and when operated in unison, acceleration. Another
option would have the three as smaller orienting devices, with one big
piece right at the center of mass for main propulsion.
Then I started wondering why one would use diamagnetic
material rather than actual magnets. Magnets can either repel or
attract, giving them better navigational and accelerational
characteristics. Furthermore, diamagnetism is a pretty weak force,
whereas supermagnets would be very powerful for maximum drive. To "turn
them off" either there could be a magnetic shield moved into place
around the magnet, or they could rotate slowly but continuously to
cancel out their effects. (The latter is simpler, but on a long flight
might tend to wear out the drive motors. In practice, acceleration
would be desired for the first half of the voyage and deceleration for
the second half, so the need to spin the magnets would be minimal at
least until the destination was reached.)
Consider a spacecraft as it orbits across Earth's north
magnetic pole. Before reaching the pole, it turns its 'south' magnetic
face to it. This is attracted, pulling the spacecraft faster. After
passing the pole, the 'north' faces of the magnets are facing the pole,
so it
pushes the spacecraft faster. When it reaches the magnetic equator, it
can turn the magnets around to gain the same effect from the south
magnetic pole.
The possibilities for navigation by orientation of the
magnets at different points of the orbit are endless. Essentially, a
spacecraft, once launched into low Earth orbit, can go anywhere - the
whole solar system and beyond.
The required continually changing magnet orientations to
accomplish
this are a bit complex to figure out. But that's what computers
are for.
NASA has apparently
considered and (for now)
essentially rejected magnetic drive. They consider it would only work
effectively in the vicinity of the Earth, and that it wouldn't be
feasible to use magnets to repel because of their instability - their
strong tendency to flip around and attract. But this last is puzzling.
With computer control of the magnet orientation and
stabilization,
and suitable design of the spacecraft, the stability is certainly
available. Blocky Stealth bombers fly - with far more rapid computer
adjustment of the control surfaces than spacecraft magnets would need.
I
think NASA is only
considering one fixed magnet instead of three, and thinking more about
magnetically accelerating stray space particles than about using
planetary fields and the solar field. In the solar field, we are
fortunate in that the sun's equator and magnetic field tilt are way off
from the plane of the solar system, getting us half way into a "polar"
orbit of the sun while in the solar system plane.
My whimsical idea for the
present satellite mission would be to test it by having it orient
itself, start and then stop spinning, shift into a polar orbit, and
then gradually make its orbit more and more elliptical by accelerating
over the north pole on each orbit, until the orbit intersects the
moon's - then crash it into the moon for a finale.
A more interesting future mission would be to send a
lightweight solar powered camera probe to Jupiter and explore its
orbiting worlds, which are IMHO much more interesting than Jupiter
itself. (See my several solar system space writings especially
including Ganymede and Callisto at http://www.saers.com/recorder/craig/)
Jupiter's powerful field should
provide for excellent navigation. The
craft could drop into orbit around each world, map it, drop down close
and get some very close-up images, then leave orbit and go to the next
one. (It could then even return to Earth, tho I'm not sure what the
point of that would be.)
Supermagnets hanging ~.1" farther south than
when oriented facing the other direction.
 A test with
supermagnets hanging from an 8 foot thread indicated at least .001g
force pushing north or pulling south at Victoria BC - the magnets hung
about .1 inch offset with the magnets one way around versus the other.
(The test was repeated at another location
just in case the results might have been influenced by local effects in
the immediate vicinity.)
A test with
supermagnets hanging from an 8 foot thread indicated at least .001g
force pushing north or pulling south at Victoria BC - the magnets hung
about .1 inch offset with the magnets one way around versus the other.
(The test was repeated at another location
just in case the results might have been influenced by local effects in
the immediate vicinity.)
The force would
increase in strength towards the magnetic poles.
Escaping from the
Earth requires that
twice the kinetic energy be imparted to the craft than to reach low
Earth orbit. With rockets, it takes a lot of fuel to to send up the
rocket
with the additional fuel as well as the craft itself, so the rocket
required is much more than twice
the size.
However, launched
into low Earth orbit, even at .001g, where a spacecraft would take
almost 20 minutes to accelerate only to the extent of falling for one
second at the Earth's surface, the minutes, hours and days start adding
up to acceleration to take the craft out of Earth orbit using the small
rocket for launch, without any additional fuel.
From relatively weak diamagnetic forces to relatively
strong supermagnet forces, the idea goes from theoretical but probably
impractical to probably workable - but I won't pretend to have worked
this
out in detail.
Next in line is obviously to use superconductors to
generate extreme magnetic fields. Perhaps the acceleration would then
actually be felt by a crew on a manned craft. With a few hundred times
the force of the supermagnet test, the craft might even lift off from
the Earth at a magnetic pole - no rockets. In the most credible reports
of UFO's (or least incredible, depending on your viewpoint), witnesses
often described puzzling phenomena that would be explained by powerful
magnetic forces. "The compass went wild." or similar words were the
most common observation, followed by failure of electromagnetic systems
such as car ignitions.
So I started looking up substances like lithium, beryllium
and boron, that have interesting properties in this regard. Surprise!:
even ordinarily beryllium is more conductive than copper or silver by
weight. Being
less dense, it isn't more conductive by volume, so the wire
would be a larger diameter, yet lighter. It was once reported (alas
incorrectly)
that room temperature superconductors had been created from them. In
fact, they'd still need liquid nitrogen to cool them enough to test.
However, once in space, by shading the coils and radiating heat,
they could probably be cooled sufficiently without any liquid. The dark
side of Mercury before dawn is the coldest place this side of Uranus or
Neptune in spite of its proximity to the sun.
Another substance that came to my attention was needle
crystals of bismuth. The individual crystals are evidently
superconducting up to 15ºC! (Bismuth is also diamagnetic. So are
superconductors.) They
can be arranged into a packet that's superconducting in one direction
and
an insulator in any other - a very interesting property. I don't know
whether growing appropriate individual bismuth crystals to make magnet
coils is feasible or not.
If I'm the one doing experiments, the
beryllium/lithium/boron in liquid nitrogen sounds more likely to be
successful.
Out of the blue on the 26th, someone sent me a link to an
MIT Technology Review
story about superconductivity research in Japan. It seems that
superconductivity can be induced in iron teluride-sulfide if you
add red wine to it and heat it. Various brands and flavors had varying
effect - even beer worked. However, in the comments someone mentioned
that the superconductivity occurred at 2ºK. Good grief!
But also in the comments was a link to more interesting
recent (Dec 2011) stuff: room temperature partial superconductivity in
a substance built in layers. Whatever was working transistioned at
301ºK (28ºC), but only parts of the material were
superconductive. So it's not ready for application, but it was the
first time anyone had had to heat a
substance instead of cool it to find the superconduction transition
temperature. IIRC, I recall that "higher" temperature superconductors
were first found around 1986. I'm sure many were as excited as I was
that
they'd soon be up to room temperature.
The link was: www.superconductors.org/28c_rtsc.htm
, and without the "/28c_rtsc.htm" on the end, it appeared to be
the
mother lode for superconductor info.
Magnetic Free Energy?
I've always been skeptical of "zero point energy" and
"perpetual motion". Whenever anyone says they have it, I'm just as much
in the dark after reading their stuff as I was before. The only ones
that even seem to come to a logical point are those that say they don't
know
how it's done because the US government has suppressed it.
But on the 31st, I considered that a if magnet facing
north is being pulled south (or vise versa), while a magnet facing the
other direction is pushed the other way, there's potential energy in
there. Okay, that could propel a spacecraft. What about on Earth?
If the two magnets were on the east and west sides of a
rotor, there would be a net torque being applied to that rotor.
Furthermore both north faces (or both south faces) were facing forward:
it wouldn't even be necessary that the magnets be movable, they could
be fixed to the rotor. The rotor could be oriented horizontally or
vertically north-south and, if friction were overcome, it would still
turn.
The idea seemed incredibly simple, and I decided to test
it at once. I used 3, 6 and then 9 magnets sandwiched between two 11"
PP-epoxy rotors (Hubcap motor body parts with holes spaced for 9
coils), with a plastic center piece balanced on the point of a center
punch. It didn't work. But
when I brought it down to two magnets facing the same way and it
wouldn't even behave like a compass and point north, I decided the
rotors must have too much weight and friction. (With three magnets it
sort of faced north.)
Perhaps I'll try making a large diameter, lightweight
aluminum assembly, and hang it from a thread?
On the other hand, the twisting force trying to point the
magnet north is much stronger than the linear motive force. Perhaps by
allowing the magnets to pivot through part of their
rotation, some continuous non-zero twisting force could be had? All
this wouldn't be "perpetual motion", tho that would appear to be the
effect -- the energy would be actually derived from the Earth's
magnetic field.
I came up with a seemingly workable design on paper the
night of April 2nd, but I have little faith that I could make it work,
especially
without making it into a major project. However, I'm no longer sure it
can't be done, and in fact pretty sure it can. Whether it could prove
to be as practical a source of free energy as solar, wind, waves,
tides,
running water or geothermal heat is questionable. But it would run
"24-7", which is a good plus. It would doubtless need a lot of
development. Again it just might boil down to working with
huge magnetic fields from superconductors.

Magnetic Impulse Torque Converter Project
I kept feeling that the
torque converter as made in January wasn't going to move the Sprint car
even if I got the springs working right, and I decided to
do the motorcycle first. It finally occurred to me what the problem is:
the "hammer rotor", despite the copper wedge and the counterweight,
seems too light. The hits it makes against the output shaft will
probably be insufficient to budge it.
If that rotor was much heavier, then when the magnet rotor
on the motor 'picks it up' - starts it turning, soon reaching almost
the speed of the motor - it will take much more inertial force to do
so, which would then be imparted to the output shaft rapidly as it
hits its end stop. Hopefully as weight is added, the magnetic
interaction will still be able to get it going and it won't slow the
motor too much.
Meanwhile, I had got the motorbike version, magnetic
impulse only, underway.
I took apart the motor from the dirt bike to change the
shaft, and on the 21st I finally cut a new shaft and turned it down to
fit the "idler gear" that was to have the the aluminum rotor with the
copper wedge bolted to its side.
Putting in a new shaft and then a couple of rotors and a
gear onto it sounded
simple enough, but a few "ten
minute" work items stretched into days. I won't go into the gory
details. On the 30th I made a little video of the unit on
the bench, which showed the magnetic pickup and the 'hammer hits'
idea.
 After
uploading the video I put it on the bike. Two nuts
protruded into the path of the chain, and because the sprocket was
bigger, the chain was too short. (True, both these things could have
been foreseen. Sometimes it would save me trouble if I was more
methodical and planned the details more carefully.)
After
uploading the video I put it on the bike. Two nuts
protruded into the path of the chain, and because the sprocket was
bigger, the chain was too short. (True, both these things could have
been foreseen. Sometimes it would save me trouble if I was more
methodical and planned the details more carefully.)
The alignment of the motor also became quite critical with
the arm (holding the copper wedge) swinging around right next to the
sprocket - it was right next to the chain along its entire length, not
just by the sprocket. I decided there needed to be more space between
the arm and the sprocket. Then the sunny break in which I was working
lifted and it went back to raining. In the shop I replaced the
protruding nuts with larger, shorter flat head screws, threading the
copper so they could screw in without protruding on the copper side,
either.
The next day I went to buy a few extra links of chain. I
discovered that while the new sprocket was #40 chain, the bike was
slightly different, #420, and the chain would only wrap about 1/3
of the way around the sprocket before it started riding up on the
teeth. Since the new sprocket with the bearing was a special part for
the converter, it was the rest of the bike that had to be redone. Back
to Princess Auto. I found #40 chain and a 60 tooth, #40 sprocket for
the back wheel. (60/17=3.5 x reduction - better than the 54 teeth of
the original.) Of course the center hole was too small, and six bolt
holes had to be drilled. I got it done, but that ended the month.
Still, on April 1 and 2 I got
everything cludjed onto the bike and tried it. The magnetic pulses of
force were surprisingly weak and only got a bit of a ride with a slight
downhill slope contributing half the force. Even for the bike this
purely magnetic impulse converter needed more magnets and copper
wedges. Mind you, I only put on one bank of battery sticks,
considerably limiting the motor current, and the motor didn't seem
to be running properly either - it ran better in one direction
(unfortunately backwards) than the other, probably indicative of having
a couple of
phase wires reversed. (I should have fixed that on the bench.) As an
alternative to multiple rotors, longer
wedges extending both the strength and the
length of the pulses (they seemed too short) might well have helped,
ie, wedges and magnets
extending 1/4 or even 1/3 of the way around the rotors instead of
1/5th. This would reduce the maximum torque gain to 4 or 3 or less
instead of near 5 - still sufficient - but with somewhat more, and
longer applied, force. I may try this sometime.
But in the store there had been some other new
parts besides the 60 tooth sprockets: centrifugal clutches. One had a
10 tooth sprocket for #40
chain. Here was something: with 10 teeth and the 60 now on the wheel, a
6 to 1 speed reduction, I think the bike should go even without a
variable torque converter. I had wanted to try this ratio (or
even higher) for a long time, but these new parts were the first
sprocket pair that I'd found that would give more than 4 to 1 -- and it
added the centrifugal clutch as a bonus.
I decided to try a whole new approach using it, even if it meant gears
instead of a
variable converter. On April 3rd I went back and bought one.
Anything useful the centrifugal clutch
did for making unsymmetrical forces with strong and weak points instead
of constant torque with no higher peaks would be an added bonus. In
essence, my last,
unbuilt, design before I tried switching to magnetic was a centrifugal
clutch that would alternately catch and bounce, rumbling along with low
losses rather than slipping smoothly along the drum and making heat. I
can play a
bit with this one. It's not quite the right shaft size, and I can mount
either the input or the output slightly off center. If, for example,
two shoes were removed and the output drum was off center, the shoes
would hit the "high spot" on the drum and then retract again, giving
something of a pulsing torque effect. And as the wheels get turning,
the clutch will grab hold and give the desired direct coupling.
I got the clutch installed on April 4th, with the
off-center output but without removing any shoes. It looked good. But
this newsletter was already past due, and the story will have to wait
for next month. (Hmm... I hope the slightly off-center sprocket isn't a
problem!)
I also took another look at a more complex washing machine
clutch setup I'd
been given earlier.
It had 3 sets of centrifugal shoes in two concentric drums. One pair
grabbed the inner drum when the motor was going fast enough. One set
worked backwards, the outer drum releasing the inner one at a set outer
drum RPM
instead of
grabbing it. A control solenoid could stop a free-spinning third set of
double in and out shoes or release it to spin - when spinning it would
activate and again tie the inner to the outer drum regardless of the
release of the backwards set. Could this make some sort of multi-speed
transmission?
Washing machines agitate clothes slowly, repeatedly reversing direction
evidently with a lot of torque. Then they spin the clothes, gradually
attaining a high speed - all from a single speed motor with no gears.
Isn't good
start-up torque and good highway speed without too high a motor RPM
exactly what was wanted? Could a larger version work for a car? But I
think
it mainly works by allowing things to slip to attain specific speeds.
That would never do for an electric car - but some of the ideas might
be useful.
A plan starts to form for the Sprint car: put the
planetary gear on the motor. 2.8 to 1, times 4 to 1 from the chain
drive to the differential, gives 11.2 to 1. If the motor provides 9 or
10 foot-pounds, that's over 100 at the wheels - that should be
sufficient, but the top speed will be only about 20 Km/Hr. Now: just
below that speed, have a solenoid release "brake shoes" holding the
body of the planetary gear. It will start to spin freely and the wheels
won't be driven. Then have shoes on that spinning body that grab the
planets assembly. Or something to that effect. The planetary gear then
locks up and starts turning 1 to 1 instead of 2.8 to one (leaving the 4
to 1 chain), and the car, with 40 foot-pounds torque, can accelerate
from 20 to about 55 Km/Hr. Good enough for a city only car in Victoria.
NiMH Battery Project
Shrinking the packages: custom
diameter thin tubes
I tried some of the "quintos" battery sticks in a friend's
24 volt lawnmower. But I could only fit in one set - 10 amp-hours. The
two
original compact lead-acid batteries (one was dead, after only 3 years)
were 20. Even with
two sets the weight would have been cut from 30 pounds to 18 and the
batteries should of course last many years... but I couldn't fit them
in. Earlier a friend had found the requisite PVC tubes wouldn't fit
into the battery space in his electric bicycle. They always seemed to
take up more space than they should, and this sometimes matters. And
the quintos weighed extra, 4-1/2 pounds instead of 4 (per 12 V, 10 AH
bank). A smaller tube would help a bit. (Considering that the
equivalent battery in AA size in a light case is only 3 pounds, we're
really losing energy density here!)
I started thinking, 20 AH at 24 volts needed 40 D cells.
At 9$ each, that would be 360$ - ouch! That just seemed like way too
much to ask. I bought a whole gas mower a couple of years ago for under
150$. But I didn't get the job done, and my friend's lawn was
growing. He bought one new lead-acid cell, and it came to 100$! (I
thought they'd be half that.) Replacing both as recommended would thus
be 200$. The NiMH D cells thus are seen as an economy: less than twice
the price, and they would last many times longer.
Another consideration was marketability. I'd only managed
to sell one car battery in a year, and that was a soldered one, before
the battery sticks. If the sticks were smaller, they'd look more
impressive under the hood.
With only one bank of dry cells, the mower ran 'in the
yellow' - slightly low voltage - since it drew 20 amps, and more
when it hit tall grass. Not a surprise. I noticed too that the cells
were warm (as well as drained) after around 10-15 minutes of mowing.
This was concerning not in itself, but for the 70 amp-hour "super
battery sticks", with 7 rows of cells in one tube. They might get quite
hot if asked to drain as fast in a car, eg going up an extended hill,
maybe on a highway.
At least they should have the center column, which would get hottest,
replaced by a ventilation tube through the middle, leaving 60 AH. ...or
should they be eliminated entirely and replaced with smaller sets?
 On the morning
of the 30th, I dug out some pieces of 1/16"
ABS plastic, heated them in the oven, and rolled them into tubes. My
vacuum cleaner wand was just the right size to roll them around. (Later
I found a 5' aluminum pipe exactly the same diameter.) After
a couple of tries, I found the best plastic piece size for quintos was
about 6-1/8" x 4-5/8", leaving abut a 1/2" seam to glue the tube
together. (Since the sheets come in multiples of 12" sizes, 6" would be
close enough for the length.)
On the morning
of the 30th, I dug out some pieces of 1/16"
ABS plastic, heated them in the oven, and rolled them into tubes. My
vacuum cleaner wand was just the right size to roll them around. (Later
I found a 5' aluminum pipe exactly the same diameter.) After
a couple of tries, I found the best plastic piece size for quintos was
about 6-1/8" x 4-5/8", leaving abut a 1/2" seam to glue the tube
together. (Since the sheets come in multiples of 12" sizes, 6" would be
close enough for the length.)
Although they were rather 'lumpy', they fit inside the PVC
tubes. Five tubes in a row occupied 7.4". Five of the old ones
were 8.5". Checking the mower battery case it looked like they should
fit exactly - four sets of five for 24 V, 20 AH.
Then I considered that one could make a single flat oval
tube, for example five cells wide by one tall and have the quintos all
in one minimal size and weight package. ...or maybe 3 x 5 and have a 30
cell car starter battery ...or perhaps 1 x 4 x 24" for 40 AH x 12 V
electric car batteries.
I ordered and got plastic to make more and looked for a
hole saw
the right size. I tried making an 1x5 by two 'D' cells oval to have
'quintos' in one container. My main conclusion is that I need some
better jigs to shape the pieces more exactly, because they look ugly
and misshapen. Or cut and glue together square cases without heating
and bending the plastic. (Wait... aren't they supposed to be battery
"sticks"?)
Turquoise Battery Project

Small diameter 1/4" pocket electrodes from late February.
3 zinc coated wire electrodes were twisted together - two corroded off -
doubtless bending them to solder them to a zinc coated nail made a gap
in the coating.
The cell worked much better with just the remaining one.
Effects of toluene on graphite conductivity
I found an interesting test for this: dipping grafpoxied
wires into toluene.
For the first wire, contact resistances to a meter probe
before dipping measured in the range of 100+ ohms. After it had
evaporated,
it seemed about the same... or were a few more of the readings a little
lower? Then I dipped a wax one that was around 4000 ohms. It seemed to
me it was a little lower - more readings around 2000, but I still
wasn't sure. So I dipped another wax one that was upper 100's of ohms
near the tip and over 1000 higher up. It didn't seem to change much -
it might have been down a bit.
Since you get a different reading every time you touch the
meter probe, it's pretty hard to tell.
Then I thought to leave one in toluene longer instead of
just dipping it in. Before, it was 150-220Ω in the good spots. After,
it seemed to me most readings were under 200.
All in all, toluene does seem to help to an extent, but it
seems to me that leaving it in longer has little or no extra benefit.
(I also noticed for the first time that many of the 4"
wires were better near the tip... When I coated them, they were
vertical, sticking up from holes in a piece of wood. I guess the epoxy
flowed
down a bit, leaving more graphite particle concentration at the top.)
Since I'm pouring a little toluene into finished
electrodes anyway (when I remember to) for the benefit of the graphite
powder, there's no need for dipping the wires in separately in advance.
But the test helped quantify the extent - maybe 3/4 or 2/3 of the
original resistance - of
the helpful effect of toluene.
I wonder if the Diesel Kleen is better?
Faster Perforating
A concern I had considering using a laser to cut and
perforate
the electrode plastic pieces was that it might take a few seconds to
burn each perforation where the sewing machine made perhaps 7-10 gashes
per
second. Even tho the machine would operate unattended, it could be slow
going.
Then I got the idea that as many laser diodes as desired
could be spaced one electrode piece width or length apart, and make as
many pieces at once as desired. The speed, whatever it was, could be
multiplied by any small integer, perhaps as much as 16 - 4 x 4
perforated electrode covers at once, if the production was too slow
with one. My order had three laser diodes and two collimating lenses,
but I didn't get around to getting it all set up and programmed.
What, Manganese on BOTH Sides?
Duh! It's amazing how we humans can pursue "not the best" down an
intriguingly complex path trying to make it work, or work better, and
completely miss better but simple things. I've
worked hard to get neutral pH cells, partly so nickel could have the
highest voltage (+1), so the cell could have higher energy density by
virtue of having higher voltage. But nickel as manganate is NiMn2O4.
It performs better than Ni(OH)2, but it literally contains two MnO2's,
and 2/3 of the
weight of a third, and yet gives the electrochemical benefit of
only one
nickel. At best, it moves about 1.75 electrons per reaction. 2-2/3
MnO2's moves probably 2-2/3 electrons in discharging fromMnO2 to MnOOH
or Mn2O3. So I started thinking about MnO2, the other common
positrode substance besides nickel. Just as I finished up last month's
newsletter, some of what I myself had said in it started to connect. I
began to realize that the "problems" with using manganese are largely
illusory or inapplicable:
* It's been said that MnZn 'renewable' cells have to be charged
carefully to prevent charging the Mn to a higher oxide. ...but first,
it doesn't really look like it should be a problem anyway, and second,
if it is, it just means needing a charger made for the type of cell
(which is hardly unusual). At neutral
pH it would have to be more severely overcharged to become KMnO4. In
fact,
at pH 8 to 13 there isn't a soluble form in
sight, and at 7, only if the cell is discharged to nothing. (In the
standard dry cell, it's the dissolving zinc side that won't effectively
recharge,
not the manganese.)
Besides... would having it charge to potassium permangante
actually be a problem? It's little soluble, and it has a higher
voltage.
* In alkaline cells, MnO2 is only +.15 volts - seems rather
pathetic - but at neutral
pH, it's +.5. In fact, having higher amp-hours per kilogram, it has
11% higher theoretical energy density than nickel hydroxide at +.5
volts in alkaline batteries.
(...in fact, 42% more if it charges and discharges fully, since Ni(OH)2
effectively only
discharges 3/4 of the way.)
When I later made a cell, it turned alkaline, and it
charged to manganate or permanganate for a cell voltage well over 2
volts, so it had high
voltage and high amp hours.
On the other hand, the advantages
of MnO2 (theoretical before making the cell) make an impressive list:
* The voltage (at pH 7) is only 1/2 that of nickel, but considering all
the additives nickel needs the amp-hours per
kilogram are probably almost double, so the energy density is actually
nearly as high.
* Double amp-hours in the positrode means double the matching
negatrode, which
(as I noted) is where the high energy density is. Therefore, doubling
the amp-hours increases the overall
energy density much more than doubling the voltage. Even if the
voltage was zero, double the amp-hours would still mean more energy.
(The Mn negatrode is 1150 WH/g of Mn; The positrodes are only around
.1 to .15
WH/g of MnO2 or Ni(OH)2.)
* It's even more conductive than [my] nickel manganate (& far more
than nickel hydroxide) for high current capacity. Especially it'll make
better performing DIY cells - which for DIY can make the difference
between frustration and success.
* It's cheap - the main ingredient of the whole battery is the same one
as
for throwaway dry cells.
* Using dry cell MnO2/graphite mix, the ratio of identical size
electrodes is exactly two positrodes for each negatrode, since the
positrode reaction moves one electron per Mn atom, the negatrode moves
two, and the starting substance is the same. It was looking
like the nickel would need 4 or 5 to 1. (So much for my alternating
electrode "checkerboard" pattern!) 5 to 1 would be six electrodes total
instead of three with MnMn - nearly double the size and weight for a
cell having only 1/3 more energy by virtue of higher voltage.
* It has low self discharge. (Like the standard dry cell.)
The cells will be only about 1.5 or 1.6
nominal volts instead of about 2 volts, theoretical open circuit
voltage being about 1.68. More cells will be needed to
attain a given
voltage. But those cells will each be little more than 1/2 the size for
the same amp-hours capacity. 3/4 the voltage but double the capacity is
1.5 times greater energy density in each cell.
It all seemd very simple until I made a cell. It seemed to charge fine
to manganate or permanganate rather than just to dioxide. But there was
the nagging possibility that the cycle life of the cell would be short
owing to the slightly soluble ions. Anyway, as I was considering the
cell...
MnO2 (+) theoretical capacity: 307 AH/Kg.
At +.5 V, that's 154 WH/Kg.
Mn (-, metal) theoretical capacity: 976 AH/Kg.
At -1.18 V, that's 1151 WH/Kg.
The relative weights:
2 positrodes * 87 = 174
1 negatrode * 55 = 55
total: 174 + 55 = 229
Energy:
(174 * 154 + 55 * 1151) / 229 = 393 WH/Kg
Of course, the cell also needs water, graphite, plastic,
etc, and these
are theoretical maximum values. Still, all the other stuff should only
double the weight... at least for factory made cells... leaving it very
close to 200 WH/Kg, a figure fit to drool over. But even if the weight
was tripled in DIY cells, 131 WH/Kg is hardly second to
lithium ion at 140 WH/Kg when it's 1/10th the price, if that!
Once I got into it, I realized it had even more energy
potential than was obvious. Having discharged to Mn2O3 or MnOOH at
valence 3, Mn can further discharge to Mn3O4 at valence '2.5' at .4
volts lower voltage. If the equipment being run can handle that, that's
another 50% longer running time at, eg, 1.2 volts instead of 1.6 volts,
simply by adding 50% more of the lightweight negative side. Even that
isn't the ultimate limit, because the Mn3O4 can discharge to Mn(OH)2,
valence 2, with another voltage drop of about .25 volts, cell voltage
under 1.0. That's the ultimate limit because the negative side also
discharges to Mn(OH)2. This doubles the amp hours, tho the energy is
less than doubled because of the lower voltages, for less than 25% more
cell weight. Theoretically (without working it out in detail) it would
be around 700 WH/Kg.
At 1.6 volts nominal, 22 cells would be needed to get
(about) 36 volts. At 1.2 volts, the battery would be down to 26 volts,
and at .95, 21 volts. The user would be alerted to the fact that a
recharge was needed and yet would have considerable reserve capacity.
Of course, a 36 volt motor running on 26 or 21 volts will
be underpowered and the top speed will be considerably lower. But I
think having at least enough negative to allow the 26 volt range would
be well worth the extra 10% weight. Voltages would stay higher towards
the end of the 36 volt range, and currents would be improved by the
higher content of metallic Mn towards the end of charge. And there's
that emergency reserve at 26 volts...
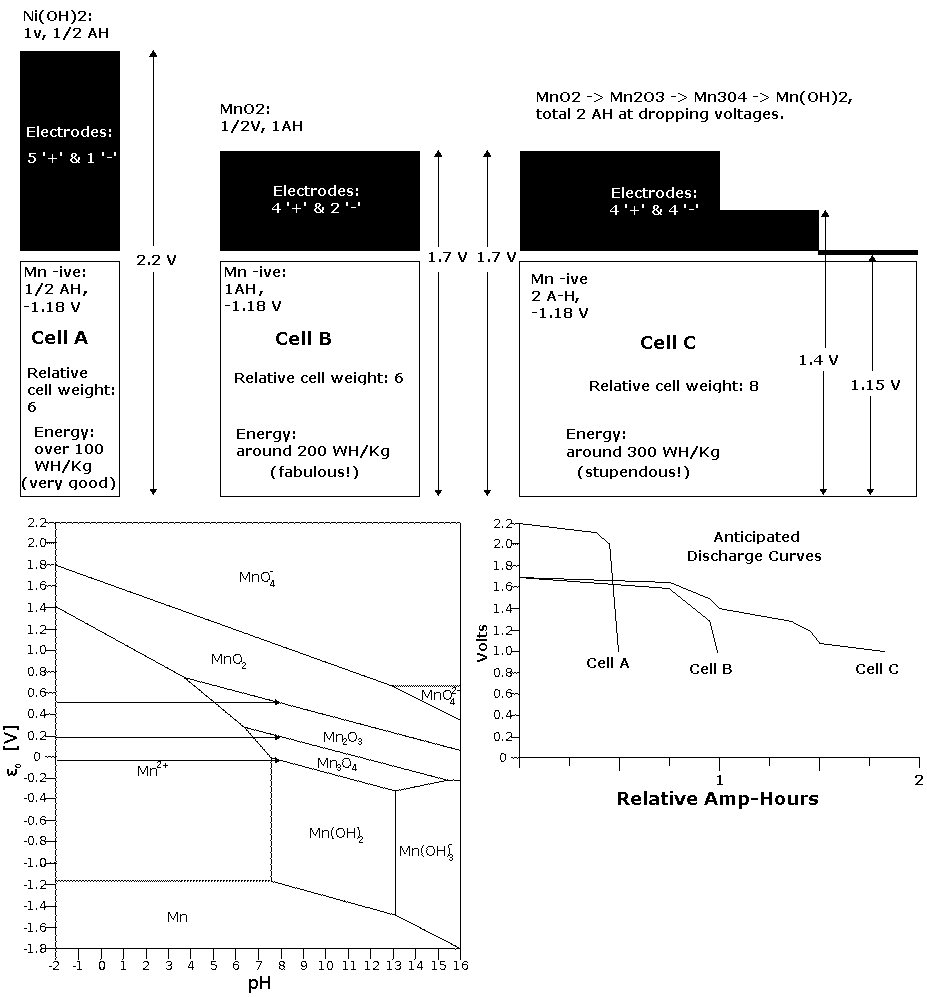
This was drawn before realizing that the positive electrode would
charge to permanganate,
giving both the high voltage of nickel and very high amp-hours.
With that, 3 negatrodes should match each 2 positrodes - Wow!
(or maybe about 1 to 1 without graphite in the negatrode.)
First MnMn Cell Tries
MnMn looked far too promising to not make a cell or
two and try it out. They worked well, and had far less self
discharge than any of my previous ones - they were actually usable
except for
having very low current capacity. At least 5 up to 50 times as much
would be a big help.
The voltage, especially of the second cell, was higher
than
expected, too. In theory at neutral pH it should be +.5 - -1.18 = 1.68
volts, or in alkaline at best +.15 - -1.56 = 1.71 volts. After some
charging, it gradually drifted down from about 2.2 volts, and it seemed
to sit at, and to recover to after a load test, over 1.8 volts. Well, I
wasn't complaining. Then it occurred to me that the positrode must be
charging to potassium manganate or permanganate after all.
The pH was strongly alkaline at 14. For the positive I
used a negative I'd been using against a nickel positive in KOH (after
pulling out the zinc current collector and inserting a grafpoxy one),
and tho I tried to dilute it out several times, it probably still had
some in there. I'll have to make a completely fresh one and see where
it goes - the pH of the first one was lower. But it too might have
turned alkaline over time.
Pure Mn Negatrode
I had been using dry cell manganese with graphite for the
first few 'trodes. On re-reading part of the Tehran paper, I noticed it
talked about conductivity additives for zinc electrodes:
"Other additives such as graphite and metal powder can act as
electronic contacts between the zinc particles. Some of these may cause
passivity, gassing and/or lowering of the specific energy."
Perhaps that's why Alkaline Storage Batteries said
'pure' zinc electrodes are made, with no
conductivity
enhancer? Unfortunately, that book says what's done but rarely gives
reasons. Could the remaining self discharge be attributed to
the graphite powder? Grafpoxy current collectors certainly seemed to
gas and be
unusable. In fact, a purpose of the "optalloy" was as a
powder conductivity additive to the electrode as well as a current
collector.
There was however a favorable favor: the stibnite, even if it didn't
fix a grafpoxy terminal post, might perhaps raise the overvoltage of
the graphite
powder sufficiently, as well as of the manganese. Was there any reason
that wouldn't
work? Later in the month, trials
seemed to show serious self discharge, tho now over days rather than
minutes or hours. By that time, one 'pure Mn' (no graphite) electrode
had failed (trouble with the zincating), and I was doing the torque
converter and didn't get around making and testing another.
In the meantime, I
was in doubt. I made a 1/2"
square cylinder negatrode with pure
MnO2, plus the 1% stibnite, 2% Veegum, and 1% Sunlight dishsoap. They
just use zinc oxide powder (+ overvoltage additive & binder) for
zinc electrodes, and the Mn does charge to metal particles, which should
be pretty conductive without graphite. I got in about 20 grams of MnO2,
about 13 theoretical amp-hours. It started off seeming like pretty
poor conductivity. The cell was somewhere around 13 ohms. Let's see...
charging a 13 amp hour electrode at
30mA should take... about 2-1/2 weeks - zounds! It actually seemed to
stop taking further charge after about 1/2 that time. But it didn't
seem to become notably more conductive.
The cell with this electrode was the first one with a
proper high voltage negatrode to have
such low self discharge that it would actually be usable as a practical
battery. (contributing to my doubts about graphite powder.) After
sitting 24 hours, the voltage was still about 2.13
volts, having dropped fairly rapidly to about 2.20, after a few hours
to 2.16, and then taking many more hours to drop to 2.13.
This electrode had one other improvement: the first
'zincated' aluminum current collector post - more on that below. That
was its second post; it had considerable self discharge with the first
one, a galvanized nail. So whether the graphite powder was causing self
discharge, or was 'fixed' by the stibnite as seemed likely, was still
in question.
Another question was why the conductivity was so low. It
didn't seem there was much connection between the electrode substance
and this second post. That was certainly something that needed
improvement.
A thought for an entirely different conductivity additive
struck me: Zinc has the overvoltage and (evidently, with the pure zinc
electrodes) the conductivity, and it discharges to oxide at a bit lower
voltage than the manganese. That means zinc powder should remain in its
most conductive metallic state as long as the Mn isn't totally
discharged, and so it should work better than it does in a zinc
negatrode. Perhaps Zn should replace graphite in the negatrode?
To continue the story of this electrode somewhat out of
sequence, I added some zinc chloride to the electrolyte. It was
slightly acidic (having been made from ZnO + HCl). After a day of
charging at low current, the voltage was almost 3 volts and the pH was
about neutral. When the charge was removed, it dropped down to 2.52
volts. If I lifted the electrode out part way, the voltage went up, and
it reduced when it was lowered again. So I put in a piece of separator
paper. Then it stayed at 2.56 volts.
It would scarcely drive a few milliamps, tho, before
dropping down rapidly to under a volt. When the load was removed, the
voltage went back up to
about the same rather perplexing figure.
By measuring
each electrode against the voltage of the water itself, it became
apparent that about half the voltage was from each electrode, and
further that the negatrode was mainly the one that wouldn't conduct
current. (AHA! The pocket cell
electrodes give a way to tell which electrode is doing what!) With
no load, it was about the prescribed
-1.18 volts for Mn metal in neutral pH electrolyte. The positrode was
at least as high in the other direction and didn't drop much with the
load.
I must conclude that the zinc chloride wasn't effective at
raising the conductivity of the 'pure Mn' negative. This surprised me -
I was sure it would start to plate through from the post outward and
make good connections. Instead white stuff - presumably there was too
much zinc chloride for it all to dissolve - seemed to be simply
clogging the perforations and making it worse. That left adding ZnO, or
immersing in zincate solution. The latter was the obvious option for
this existing electrode.
Negatrode with metallic Mn
Before trying the "zinc infiltration" ideas above, I
decided to make another one with some of the Mn metal
powder (7g) in it as well as dry cell MnO2/graphite (13g), to see if it
would have better conductivity. Theoretically it should have a couple
more amp-hours, but I think the center of the metal particles will
never discharge, so probably it should be about the same.
I put it in the cell. Immediate results weren't
spectacular, but then I remembered that I'd meant to pour on some
toluene to get the graphite to dissolve and reform. Oops. I took it out
and left it to dry overnight, putting the other one ("pure" MnO2) back
in to
charge more.
The next morning I poured a little toluene on the top. It
didn't seem to affect the PVC, but the ABS bottom plate became soft and
sticky. That meant the toluene had penetrated through the entire
electrode - good! I left it a few more hours for the toluene to
evaporate.
A couple of days later, I removed the top cap and checked
the actual contact resistances to the electrode material. Readings were
in the K ohms. Apparently I was setting myself up for another
conductivity failure with my careless work. I pulled out the galvanized
nail and scraped out the substance. I added more graphite powder, a bit
of Sunlight, and a little water, tamped it down in the mortar, and
checked again. Low x100's. I added graphite a couple more times. It
ended up as mid x1-'s of ohms between any two points.
I stuffed 23 grams of this into the electrode, then I took
a drill bit and dug a hole for the center post. I got 1 gram back out,
total 22 grams of some inexact mix of MnO2, surface hydrated Mn powder,
Sb2S3, Sunlight dishsoap, and Veegum.
This electrode got a 'zincated' aluminum rod also sprayed
with 'cold galvanizing spray' and sintered in the oven. This is covered
further down. This was replaced with a simple zincated one. It also
didn't get put in a cell and a few days later, the resistance readings
were [your favorite expletive here], for no evident reason.
That was about when I started to consider that
graphite probably didn't have the required overvoltage (maybe even with
the stibnite?) and might cause gassing and self discharge. If it didn't
hold a charge, that would be why.
Mn Negatrode with Zn conductivity enhancer
With the 'pure' Mn electrode having poor conductivity,
something to try was to replace graphite powder with zinc powder. If
all went
well, zinc metal powder should in fact be more conductive than graphite
powder. A down side was potential degradation of the electrode if it
was discharged so far that the zinc started turning into oxide. On the
other hand, if it only happened once or twice, it might actually
improve it.
I mixed 12g calcined ZnO, 12g pure MnO2, .3g Sb2S3, .25g
Veegum, .4g of Sunlight and a bit of water. (Toluene to dissolve
graphite seemed superfluous since there was no graphite.) After the
MnO2 converts to Mn(OH)2, the zinc should charge to Zn metal particles,
then the Mn(OH)2 should charge to Mn metal particles. After that, it's
intended that the Zn stay charged to conductive metal with a high
hydrogen overvoltage, while the Mn charges and discharges with cycling.
A 'zincated' aluminum post - evidently an effective type -
was the current collector.
The resistance readings were very high, kilohms, but the
ZnO should charge up to metallic Zn.
Probably it has substantially more Zn and more Sb2S3 than
required, and the Veegum might be unnecessary. More or less of the
dishsoap might be tried.
I went to stick it in KOH for a while to eliminate any
ZnCO3 (or MnCO3?) that might have formed. Then it occurred to me that
the zincate solution was NaOH with dissolved zinc... If I stuck the Mn
electrode in this solution instead, perhaps in addition to eliminating
carbonate, a fine film of pure zinc would be electrodeposited
throughout the electrode. If it didn't encapsulate the MnO2 entirely,
it might work better than the ZnO powder. Furthermore, if any zinc had
been scraped off the aluminum rod, it should get replaced.
So I put the electrode in a jar with a little of that
instead of KOH. Over 1/2 hour, bubbles indicated that zinc plating - or
at least some reaction - was occurring. After an hour I took it out and
put it in a jar of water to dilute out the NaOH.
Building on this theme, it occurred to me that I could use
one of zinc's notorious characteristics, that of building stringy zinc
'dendrite' crystals as it charges, to further improve internal
conductivity of the electrode. Use HCl to turn ZnO into ZnCl2, which is
somewhat soluble, and becomes (temporarily) part of the electrolyte.
When a zinc Zn++ ion hits an active bit of the negatrode as it charges,
it picks up two electrons and becomes Zn metal. This picks up more
zinc, building conductive networks through the electrode until the zinc
in the electrolyte is depleted. Again, it isn't intended to discharge
the cell to where the zinc discharges - it stays metal.
This idea seemed promising enough to try out on the
previous electrode already in a cell. The only notable effect should be
an increase in the lamentable cell conductivity - without having added
zinc directly to the mix at all. It seemed to give it some self
discharge. The new electrode still had huge resistances after the
zincate bath. Nothing to do but try it out...
Next came a new battery case and a matching positrode. I
used dry cell MnO2 (20g) with a bit of Sunlight for binder (.35g) and a
bit of Nd2O3 (.5g) to raise the oxygen overvoltage. I compacted in
about 15g of this mix. As I was shoving in the grafpoxied copper wire
post, when it was pretty much in it suddenly kinked, exposing bare
copper. I melted on some graphited wax, which then also formed the top
cap. I guess the toluene would have had to be introduced through the
perforations... but I forgot it.
The cell started at 1.4 volts - after all, it was dry cell MnO2,
probably mostly charged, and there was charged metallic Mn in the
negative. Conductivity was poor, and putting one meter probe in the
water indicated it was mostly the negative that was the problem. But as
it started to charge, the 2.25 volt charging voltage reading gradually
started dropping, even while the off-charge voltage was going up - the
zinc was charging to metal and increasing the conductivity. Gosh, for
once one of my plans seemed to be working! Off charge voltage was
quickly getting up around 1.9 volts - usually that took a day of
charging, while on charge dropped to 2.18. Albeit, the current in all
this was only about 20 mA.
After dousing a second negatrode in 'zincate', I found
that the voltage dropped lower after charging than it had previously,
it still had poor conductivity, and the voltage dropped rapidly under
load. Then I realized that the zincate covering might have been more
effective than intended... the electrode seemed to be charging only to
Zn voltage instead of to Mn, and only the superficial coating of zinc
was charging and discharging.
Further positrode substance considerations
If the pH is going to be 14 notwithstanding the neutral
KCl salt electrolyte, and as it appears the cell is charging to
manganate or permanganate anion, the question comes up as to what the
cation is going to be. Potassium permanganate is slightly soluble - I'm
not sure about potassium manganate.
I suspect nickel manganate - the very substance I was
making last month - isn't soluble. I'm confused by its formula,
supposedly NiMn2O4 instead of NiMnO4, when it's Ni++ and the
manganate ion is MnO4--. Could someone have written it wrong and
everybody else just copied it? Certainly that sort of thing happens. On
the other hand, "manganate" can evidently be employed as a broader term.
If nickel manganate - at least my nickel manganate - were
actually NiMnO4 like it ought to be, perhaps the reactions would be
manganate reactions rather than nickel reactions. The nickel could
become... NiCl2?... while the MnO4-- became MnO2, moving two electrons
at about +.6 volts. (The nickel might become hydroxide or chloride.)
That might very well work! Charging to manganate would explain the
higher voltages I've been getting - about the same as with the nickel
manganate.
That would probably mean that the nickel manganate has the
same reactions as manganese, and is much better than nickel hydroxide.
Arrgh, chemistry! It's too confusing. If I can get better
conductivities I can charge electrodes up in a day instead of in weeks,
and then measure amp-hours. Then it will become more apparent
empirically what works best. I'm suspecting again that it's the nickel
manganate, and that that substance has manganese reactions and not just
nickel reactions. However, one thing that's becoming clear is that the
cells are working either way. If it's not too much extra weight (or
none), nickel manganate would probably have better (not to say
indefinite) cycle life, and so might be a better choice than straight
manganese oxides.
I decided that instead of replacing the negode of the
first cell, I'd make another one and continue charging the Mn-Mn cell.
- which after a couple of days I could hardly get any current into at
less than about 2.7 volts. The new negatrode sat idle a while.
Common thing stumbled across... I more or less remember
this from elementary school, but I don't think the teacher gave us the
numbers. Now if I can find ordinary red litmus paper, I should be able
to distingush "just high enough or better" alkaline pH from neutral,
electrode dissolving pH.
BLUE LITMUS PAPERS: Blue litmus paper turns red in an acid
solution below pH 4.5. Blue litmus paper stays blue in a base.
RED LITMUS PAPERS: Red litmus paper turns blue in an alkaline (base,
alkali) solution above pH 8.3. Red litmus paper
stays red in an acid
Negative
current collector coatings
Tin:Silver 90:10
(or was it 95:5?) Solder
I don't know if the usual stuff with 3% silver will work
(must try it), but a higher silver content solder does. An electrode
with such a current collector isn't self-discharging. To increase the
silver content, melt the silver solder in a pot on the stove. Don't get
it too hot - you definitely don't want to boil tin or zinc as the fumes
are harmful. Stir in pieces of
silver. They form an alloy with
the tin one atom thick on the surface. This alloy's melting point is
below the stove temperature, so it melts, exposing more silver surface,
and thus the silver gradually "dissolves" into the mix. The melting
point of silver is much higher than the stovetop, so at some
concentration of silver in the tin, no more will go in, and you
soldering iron might not melt it. A little copper, nickel or zinc in
the mix shouldn't hurt. (In fact, zinc helps with overvoltage. Tin-zinc
solder might work nicely too... or will it corrode? - must re-read that
Iran research paper!)
If you have 100 grams of 97:3 tin-silver solder and add
(just over) 2 grams of silver, it's 95:5, or 7 grams for 90:10. The
sluggish flow characteristics of such a solder are less important than
the fact that it will coat a metal surface and give it a high hydrogen
overvoltage.
Since your pot solder has no flux, use an electronics
store paste flux or Kester "44" resin flux, not acidic plumbing flux
(which will wreck your soldering iron tip, and any residue will
gradually eat the wire). Organochloride flux fumes are evidently
harmful to
breathe (think "Methoxychlor" insectiside), so avoid those types too if
you see them.
After writing this, I looked at a couple of tables of
hydrogen overvoltages. According to that table, silver isn't high
enough. Of the useful metals listed, only zinc (-.75 or -.77 volts) is
above about half a volt. The voltages just work: -.833 for hydrogen
itself + -.75 or -.77 for zinc = -1.58 or -1.60, and the Mn's voltage
is -1.55 or -1.56. It's possible that an alloy such as tin-silver may
be higher than either element alone, and tin is -.49 volts. But I think
I'll stick with zinc anywhere below the water line, reserving the
solder only - just possibly - for connecting negative terminal leeds
just under the top cover.
"Zincate" on Aluminum
I thought a 4" galvanized (zinc dipped) nail would be a
great current
collector. It worked (well, sort of - it was the cause of the remaining
self discharge), but it weighed 9 grams for a negatrode with 20
grams of Mn,
immediately diluting the energy density by 1/3. (The perforated plastic
shell is about 5 grams.) That's okay for off-grid energy storage, not
so good for electric transport. So I thought of
aluminum instead of steel as being much lighter for a fat current
collector wire. The density of iron/steel is 7.8, and aluminum is 2.7,
so it would only weigh 3 grams. But there are problems with using
aluminum.
At CaswellPlating[.com; caswellcanada.ca] I found "Zincate
Pretreatment" for aluminum. Per Caswell's description: Aluminum
forms an oxide layer the minute it is exposed to
air, which
prevents plating [and solder] from sticking. The
Zincate dip process chemically removes the oxide layer and at the same
time applies a layer of zinc.
A single dip that puts a layer of zinc on aluminum! - that
sounded perfect, so I ordered some. My main concern was that the layer
might be very thin and wear/scrape off as the wire was pushed into the
electrode. But I figured if that was the case, I could solve it by
using the "cold galvanizing spray" to add more zinc to the initial
coating, then heating it in the oven to help it spread and fuze. (as
with the motor rotors and other zinc primed steel parts.) Again the
zinc spray by itself wouldn't work directly on the aluminum's surface
oxide without the pretreatment.
When it arrived, I
took an aluminum rod and immersed it in the
"Zincate Solution".
When this was stuffed in the hole and pulled out, the zinc layer
appeared to have been rubbed off, as I suspected it would be. I zinced
it again, then sprayed it with "Cold Galvanizing Compound" ("97% zinc
powder" spray can), two coats, and put it in the oven at 225ºC for
20 minutes to sinter it. Magnified, the finish was very matt and porous
- sintered. Of course, none of the pores should penetrate the
'zincated' coating underneath, and nothing would get in to rub it off.
I shoved it into the electrode. Resistance readings were
interesting: around 37-60 ohms from any point to any other (depending
how hard you pressed). But when I tried to measure to the post, the
readings were bizarre - megohms or kilohms, negative or positive,
and nothing consistent at all. I locked the meter on xxx.x Ω scale and
got, eg, 220Ω with the meter probes one way, and -135Ω the other. Maybe
if the electrode was totally dry?
Giving up on measuring that, I decided to try diesel kleen
instead
of toluene. I removed the top cap and poured a little on and left it to
reek, er, soak.
Later the resistances seemed about the same. I'm not sure
it really soaked in - I wiped some liquid off the top of the cap a
couple of times. But the post didn't seem very tight. I gave it a twist
and it seemed to grab on. When I checked resistances to the post, they
were in the lower x10's of ohms - even a 9.8Ω reading. Too bad I didn't
check them before I twisted.
After charging the cell a day or so, I pulled out the rod.
It appeared that all the zinc, or at least all the 'cold galvanizing
spray', had come off. Not good!
However, I had 'zincated' another piece of aluminum rod,
and
with no overcoat I put it in the negatrode of the other cell. This cell
I'd opened, dried the electrodes, and poured some toluene in both. The
conductivity of the cell was even worse (sigh!), but the self
discharge
dropped off to virtually nothing with the zincated electrode.
Success at last!
That
would seem to mean that the thin zinc layer was intact, and also that
the galvanized nails must have had impurities or gaps that were causing
the
remaining small but vexing self discharge. I think I should complain to
the hardware store. It was a bit puzzling that evidently the abrasion
hadn't scraped off the thin zinc layer, at least in this electrode. Had
the
rubbing just polished the zinc shiny in the first one, so I thought it
was bare aluminum?
I'd know for sure in a while - exposed aluminum
disintegrates rapidly in alkali. It held, at least for the month. In
fact, that's how the 'zincate
solution' strips the oxide layer off, making aluminum hydroxide and
hydrogen - the main liquid in the solution is sodium hydroxide. The
zinc in the solution (whatever its form - maybe it really is Zn(OH)4--
zincate ion, eg, sodium zincate?) must immediately cover the exposed Al
metal with zinc, otherwise, the entire piece of aluminum would quickly
dissolve. It seemed to last. So did the next one.
Once I knew it was sodium hydroxide, I had the thought
that maybe I could make my own. What was the zinc? From
Wikipedia:
Solutions of sodium zincate may be prepared by dissolving zinc, zinc
hydroxide, or zinc
oxide in an aqueous solution of sodium hydroxide.
Simplified equations for these complex processes are:
- ZnO + H2O + 2 NaOH → Na2Zn(OH)4
This seems rather
interesting, since zinc oxide is the discharged form of a negative zinc
electrode, and zinc electrodes don't spontaneously dissolve - at least
not in KOH. Perhaps NaOH
can't be substituted for KOH in a cell with a zinc electrode, as it can
be and sometimes is
in Ni-Fe batteries. And then, why does it plate onto the aluminum and
stay? Perhaps it's forming a zinc-aluminum alloy. Anyway, it seems just
about
too simple... mix up some NaOH and pour in ZnO. I already have those.
Later I found fine aluminum grill and thin wire at Opus
Art Supplies. I knew they had coarse grill, but it was off my radar
screen as a place to find wire!
Now, if I could find something that would put an
impermeable layer of conductive carbon or something on metal for the
positive sides so easily, things would start getting pretty simple!
Terminal Post
A separate consideration,
for both electrodes, is the shape of the terminal post. With the
present technique, the electrode is compacted in advance with no post,
then a hole is dug out with a drill bit, and then the post is forced
in. With straight sides, only the bottom tip of the post is forcing
material to the side. The rest just slides in to the hole already dug,
and doesn't press against it to make good contact. A post slightly
tapered over the whole bottom 50mm that goes into the electrode would
be ideal, pushing the material out a bit all along the length to keep
it
compressed against the post.
Starting with a fat piece of
aluminum, the simplest thing to do might be to grind, file or sand it
down into a tapered shape (sand = with stationary belt sander). Then
zincate it.
For the
positrode... I'm not quite sure. Obviously there must be ways.
The other option is to go back to the plunger with the
hole in the middle and compact with the post in place. Hmm... that
might be preferable.

Terminal posts
6 positives: graphited wax, grafpoxy sucked into a plastic drinking
straw (hollow),
two grafpoxy/straw (sanded, one to a taper), one made wrapped in a
yellow paper cone (sanded),
and one painted onto a tapered plastic handle.
Negatives: 2 of heavily incated aluminum, one with zincated aluminum
screen wrapped around.
These posts were left in the zincate far too long, and didn't seem the
least bit immune to KOH.
Perforated, Zincated Aluminum Pockets?...
Al rod, Perf. Al sheet, single wrap of Al expanded mesh (1/4" x
1/2"),
3 layers of zincated mesh with zincated Al leed wire (1/2" x 1/2").
The original mesh is seen behind. None of these have been tried so far.
 But there was
another option: If aluminum can be
'zincated', why not put a zincated aluminum grill around inside the
whole shell of the electrode, or even make the whole pocket of
perforated, zincated aluminum sheet instead of plastic? That would put
a whole lot more metal closely adjacent to a whole lot more electrode
substance. Aye, there was current capacity! Surely it was the way to go.
But there was
another option: If aluminum can be
'zincated', why not put a zincated aluminum grill around inside the
whole shell of the electrode, or even make the whole pocket of
perforated, zincated aluminum sheet instead of plastic? That would put
a whole lot more metal closely adjacent to a whole lot more electrode
substance. Aye, there was current capacity! Surely it was the way to go.
The sewing machine punched through thin aluminum - not the
least bit easily or elegantly - and I made a square aluminum pocket. It
was half the weight of the plastic, and being thin with a thin bottom,
would hold a couple more grams of electrode - great for energy density.
However, I couldn't get the corner seam closed. I could burn a hole
right through with the tack welder, but it wouldn't stick together.
The next day, the 17th, I bought some fairly fine expanded
aluminum mesh and tried a few things. Finally, it seemed best to wrap
about three layers around the square steel rod , cut the corners, and
fold the ends, the lower end to form a bottom, and the upper end simply
folded over outwards.
I 'zincated' it before bending it, and after with a
zincated aluminum wire (#14 AWG - from the same art supply store)
tucked in under the outer layer. Instead of a PVC basket, it had an
aluminum one. It weighed about 4 grams. The plastic plus a fat aluminum
terminal post weighed about 9 grams. That bode well for energy density
as well as for higher current drive.
...or Zincated Aluminum Spiral wrapped Grills? -- A New Way To Make
Electrodes
Then it occurred to me that if I was winding up 2 or
3 layers of mesh, why didn't I just roll electrode paste as 'pie crust'
onto the mesh, and then roll it all up into a spiral, sort of like a
dry cell but just one lone solid electrode in it? An extra layer or two
of mesh continued around the outside would enclose it as well as the
square pocket. That way, the mesh would be distributed throughout, and
none of the substance would be more than a couple of millimeters from
some part of it. That should give the best current capacity, and it
would be much easier to do than the dry cell with two electrodes
wrapped into one coil. AFAIK, it would in fact be a new way to
construct electrodes. It could hardly be called a pocket electrode, but
the 'stand alone electrode' effect was the same.
I cut out a 70 x 100mm piece, and rolled a 100mm aluminum
leed wire into it, then zincated them. 25 grams of MnO2/graphite/Sb2S3
mix plus Sunlight and Diesel Kleen were applied to the screen and
flattened with a rolling pin. (well... with a small diameter glass
bottle.) Readings on the tamped mix were around 100 ohms. 5 grams
flaked off during the rolling up, leaving about 20 in the electrode,
same as in the pockets. There was no extra screen left to wrap around
the outside. I wrapped a
separator paper around it, put
some small cable ties around it to hold it together, and pushed it into
a case. It was a lumpy ellipse. Maybe better that way. It only weighed
about 25g total - good for
energy density even for a factory cell. In fact, the dry electrode
itself probably had (theoretically) well over 600 WH/Kg. But it's the
energetic side, and there's still the case and water to account for as
well.
The next day, the 18th, I
figured the Diesel Kleen had evaporated and I took the previous
positrode to make a cell. It seemed much better. When the charge was
removed, the voltage went down a bit and quickly stopped moving,
instead of gradually dropping for some time. With only a little
charging it would put 70 mA into 10 ohms, and keep on doing it instead
of gradually dropping off over a few minutes. However, it didn't
improve a lot with charging.
The terminal post corroded off the plus side during
charging (so much for the wax sealing the crack!) and I stuffed
another, thinner, one in. It definitely worked worse. So I pulled that
one out, and put in the best fatter one. This time there was a
significant improvement.
A load test after a few more hours charging seemed to show
that it wasn't a lot better than other recent cells, and most of the
voltage drop was from the negatrode despite expectations. It's not
getting much 'bang for the buck' either from the size of the electrodes
or from the square centimeters of interface area.
And here I thought the neg was good to go! I'm still not
sure the graphite in it doesn't cause a bit of self discharge. I'll try
the next one without graphite. Zincating the finished electrode for a
very short time instead of 30 minutes might deposit about the right
amount of conductive zinc without having it coat everything. Anyway,
more light aluminum screen and less (or no) graphite would further up
the amp-hours and energy density.
Still, the performance didn't inspire confidence.
Obviously a larger number of substantially smaller diameter winds would
put more substance closer to the positrode with more interface area,
but then you're doing a lot of work to make each small electrode. The
traditional flat screen like so many other cells use - in a plastic
pocket enclosure - might be the best way to go after all.
http://www.TurquoiseEnergy.com
Victoria BC


 At the very
end of the month, I thought of a more Earthly
application: It should be possible to get a magnet rotor to draw power
to rotate continuously from Earth's magnetic field if it was set up
right. But of the two forces - the one that wanted to twist the magnets
into magnetic alignment and the one that wanted to move them linearly,
the former was much the stronger, and that would need more than just
static magnets glued to a ring.
At the very
end of the month, I thought of a more Earthly
application: It should be possible to get a magnet rotor to draw power
to rotate continuously from Earth's magnetic field if it was set up
right. But of the two forces - the one that wanted to twist the magnets
into magnetic alignment and the one that wanted to move them linearly,
the former was much the stronger, and that would need more than just
static magnets glued to a ring. As expected,
it wasn't until late in the month that I got
back to the torque converter, to make a magnetic impulse only
implementation for the motorbike. I really wanted to get that done and
test it.
But "the devil in the details" was on his best game. The work, which
seemed simple enough in principle, proceeded painfully slowly. I
'finished' it on the 29th and made a short demo video on the morning of
the 30th, but when I put it on the bike, I found changes were needed to
get it to work.
As expected,
it wasn't until late in the month that I got
back to the torque converter, to make a magnetic impulse only
implementation for the motorbike. I really wanted to get that done and
test it.
But "the devil in the details" was on his best game. The work, which
seemed simple enough in principle, proceeded painfully slowly. I
'finished' it on the 29th and made a short demo video on the morning of
the 30th, but when I put it on the bike, I found changes were needed to
get it to work. A test with
supermagnets hanging from an 8 foot thread indicated at least .001g
force pushing north or pulling south at Victoria BC - the magnets hung
about .1 inch offset with the magnets one way around versus the other.
(The test was repeated at another location
just in case the results might have been influenced by local effects in
the immediate vicinity.)
A test with
supermagnets hanging from an 8 foot thread indicated at least .001g
force pushing north or pulling south at Victoria BC - the magnets hung
about .1 inch offset with the magnets one way around versus the other.
(The test was repeated at another location
just in case the results might have been influenced by local effects in
the immediate vicinity.)
 After
uploading the video I put it on the bike. Two nuts
protruded into the path of the chain, and because the sprocket was
bigger, the chain was too short. (True, both these things could have
been foreseen. Sometimes it would save me trouble if I was more
methodical and planned the details more carefully.)
After
uploading the video I put it on the bike. Two nuts
protruded into the path of the chain, and because the sprocket was
bigger, the chain was too short. (True, both these things could have
been foreseen. Sometimes it would save me trouble if I was more
methodical and planned the details more carefully.) On the morning
of the 30th, I dug out some pieces of 1/16"
ABS plastic, heated them in the oven, and rolled them into tubes. My
vacuum cleaner wand was just the right size to roll them around. (Later
I found a 5' aluminum pipe exactly the same diameter.) After
a couple of tries, I found the best plastic piece size for quintos was
about 6-1/8" x 4-5/8", leaving abut a 1/2" seam to glue the tube
together. (Since the sheets come in multiples of 12" sizes, 6" would be
close enough for the length.)
On the morning
of the 30th, I dug out some pieces of 1/16"
ABS plastic, heated them in the oven, and rolled them into tubes. My
vacuum cleaner wand was just the right size to roll them around. (Later
I found a 5' aluminum pipe exactly the same diameter.) After
a couple of tries, I found the best plastic piece size for quintos was
about 6-1/8" x 4-5/8", leaving abut a 1/2" seam to glue the tube
together. (Since the sheets come in multiples of 12" sizes, 6" would be
close enough for the length.)


 But there was
another option: If aluminum can be
'zincated', why not put a zincated aluminum grill around inside the
whole shell of the electrode, or even make the whole pocket of
perforated, zincated aluminum sheet instead of plastic? That would put
a whole lot more metal closely adjacent to a whole lot more electrode
substance. Aye, there was current capacity! Surely it was the way to go.
But there was
another option: If aluminum can be
'zincated', why not put a zincated aluminum grill around inside the
whole shell of the electrode, or even make the whole pocket of
perforated, zincated aluminum sheet instead of plastic? That would put
a whole lot more metal closely adjacent to a whole lot more electrode
substance. Aye, there was current capacity! Surely it was the way to go.