 If
I didn't get it in last issue, at the start of the month I mounted
the indoor radiator unit for the open loop air heat pumping on the
wall
near where the other components are. The fan runs. (Such a small
accomplishment for a
month!)
If
I didn't get it in last issue, at the start of the month I mounted
the indoor radiator unit for the open loop air heat pumping on the
wall
near where the other components are. The fan runs. (Such a small
accomplishment for a
month!)
 I
finally skinned the first half of the east wall that I had bought
interlocking metal pieces for. All except one were a few inches
longer
than they needed to be. Since the store wouldn't cut angled ends,
I had
to cut them all myself with the angle grinder anyway, and a bit
too
long is Far better than a bit too short. There really wasn't much
waste.
I
finally skinned the first half of the east wall that I had bought
interlocking metal pieces for. All except one were a few inches
longer
than they needed to be. Since the store wouldn't cut angled ends,
I had
to cut them all myself with the angle grinder anyway, and a bit
too
long is Far better than a bit too short. There really wasn't much
waste. And I
started
on the
permanent 36V DC wiring, using under the stairs as a wiring closet
and
place for the batteries. It's really nice to have two ceiling
lights
with switches by the entries.
And I
started
on the
permanent 36V DC wiring, using under the stairs as a wiring closet
and
place for the batteries. It's really nice to have two ceiling
lights
with switches by the entries. I put
the
battery under the stairs near the base. I need to make a cover for
it
before I accidently short it out with some bit of metal or wire.
I put
the
battery under the stairs near the base. I need to make a cover for
it
before I accidently short it out with some bit of metal or wire. I ran across a
video about a 1938 car, the Schlörwagen Pillbug that was
designed (by Shlör, pictured) to have an absolute minimum
coefficient of
drag (COD) or drag coefficient (DC). The lower the COD,
the
less extra fuel the vehicle needs to counter wind resistance at
higher
and higher travel speeds. Typical cars are in the .3 to .4 range.
Actual drag results from the shape and size plus skin friction. At
higher speeds it is proportional to the square of the velocity.
I ran across a
video about a 1938 car, the Schlörwagen Pillbug that was
designed (by Shlör, pictured) to have an absolute minimum
coefficient of
drag (COD) or drag coefficient (DC). The lower the COD,
the
less extra fuel the vehicle needs to counter wind resistance at
higher
and higher travel speeds. Typical cars are in the .3 to .4 range.
Actual drag results from the shape and size plus skin friction. At
higher speeds it is proportional to the square of the velocity.


 I hope
bears
don't venture out across my lawn to where the apple trees are. For
the
first time it looks like I'm going to get a good crop. This
'liberty'
tree has never had more than seven apples before.
I hope
bears
don't venture out across my lawn to where the apple trees are. For
the
first time it looks like I'm going to get a good crop. This
'liberty'
tree has never had more than seven apples before.
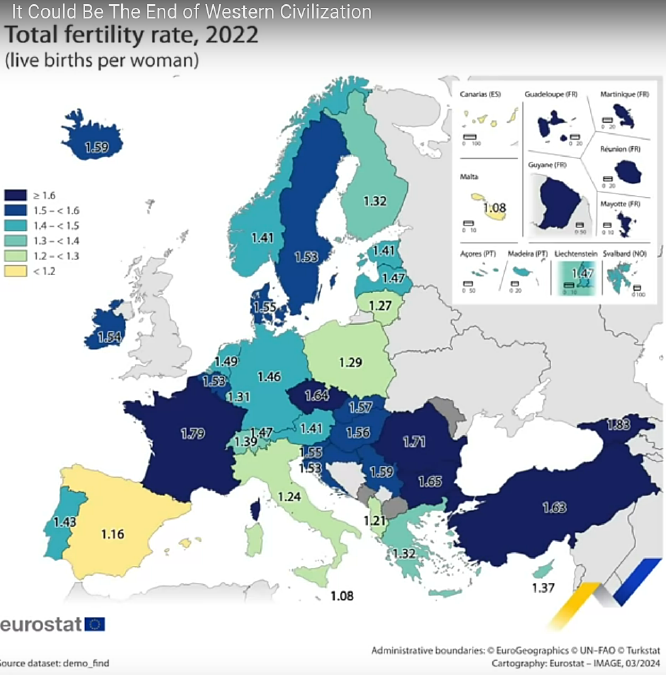 * Are the
white races
coming to an end? It takes about 2.1-2.2 children per woman to
replace
the population. For half a century we've been below that and
dropping.
If each
generation is just over half the size of the
previous, and if the incredible mass migrations into Europe and
North
America continue, will the white races not vanish faster than the
North
American red skinned natives did?
* Are the
white races
coming to an end? It takes about 2.1-2.2 children per woman to
replace
the population. For half a century we've been below that and
dropping.
If each
generation is just over half the size of the
previous, and if the incredible mass migrations into Europe and
North
America continue, will the white races not vanish faster than the
North
American red skinned natives did?








 I hope
bears
don't venture out across my lawn to where the apple trees are. For
the
first time it looks like I'm going to get a good crop. This
'liberty'
tree has never had more than seven apples before. The pear trees
are
still a complete wipeout for the eighth year. The new anjou
purchased
last year was covered with blossoms but set no fruit, and there
was
just ONE little cluster of flowers on one of of the two older
pears and
again no fruit.
I hope
bears
don't venture out across my lawn to where the apple trees are. For
the
first time it looks like I'm going to get a good crop. This
'liberty'
tree has never had more than seven apples before. The pear trees
are
still a complete wipeout for the eighth year. The new anjou
purchased
last year was covered with blossoms but set no fruit, and there
was
just ONE little cluster of flowers on one of of the two older
pears and
again no fruit.







 [2nd] Melting
holes into the PVC pipe didn't work well. If I used the
smallest pin frogs heated on an electric 'hot plate' burner, I
could
push the pipe down into the frog points, but it wasn't easy. I
thought to raise the temperature for the next try, then found that
the
lead was giving way and the pins were bent over and loose. And the
whole pipe heated up so much over the burner that it warped. So
much
for that method! If the pin frogs had a stronger base it might
have
worked - alume maybe. Lead was too soft.
[2nd] Melting
holes into the PVC pipe didn't work well. If I used the
smallest pin frogs heated on an electric 'hot plate' burner, I
could
push the pipe down into the frog points, but it wasn't easy. I
thought to raise the temperature for the next try, then found that
the
lead was giving way and the pins were bent over and loose. And the
whole pipe heated up so much over the burner that it warped. So
much
for that method! If the pin frogs had a stronger base it might
have
worked - alume maybe. Lead was too soft. What
was an
alternative? I put a new piece of pipe in an unmounted vise, in a
tray
to catch the sawdust. Then I got out the
'dremmel' tool and a tiny drill bit, maybe 1mm or so, and started
drilling holes, one at a time. It probably took 20-30 minutes to
fill
the pipe with tiny holes all the way around and along the length.
I
smoothed it off inside and out with 'scotchbrite'. (I probably
should
have used a substantially bigger bit. After all, it's the treated
paper
that holds the powder in. Small holes just restrict the flow of
electrolyte.)
What
was an
alternative? I put a new piece of pipe in an unmounted vise, in a
tray
to catch the sawdust. Then I got out the
'dremmel' tool and a tiny drill bit, maybe 1mm or so, and started
drilling holes, one at a time. It probably took 20-30 minutes to
fill
the pipe with tiny holes all the way around and along the length.
I
smoothed it off inside and out with 'scotchbrite'. (I probably
should
have used a substantially bigger bit. After all, it's the treated
paper
that holds the powder in. Small holes just restrict the flow of
electrolyte.) I
scrunched it down - farther and farther - in the
hydraulic press. By the time it was around 500Kg of pressure and
the
powder 2/3(?) of its original height, I decided that was surely
enough.
There were two problems. The first I had foreseen: I couldn't push
the
CuNi terminal/current collector into the compacted powder. But I
had
bent it into an "L" shape, much stronger than just flat, and cut
it to
a point at the bottom end. I drilled into the powder with a long
drill
bit (which went surprisingly slowly) and then pounded the terminal
into
the loosened center area. (At least, I hope it went straight in
and
didn't munch the separator paper around the edge.
I
scrunched it down - farther and farther - in the
hydraulic press. By the time it was around 500Kg of pressure and
the
powder 2/3(?) of its original height, I decided that was surely
enough.
There were two problems. The first I had foreseen: I couldn't push
the
CuNi terminal/current collector into the compacted powder. But I
had
bent it into an "L" shape, much stronger than just flat, and cut
it to
a point at the bottom end. I drilled into the powder with a long
drill
bit (which went surprisingly slowly) and then pounded the terminal
into
the loosened center area. (At least, I hope it went straight in
and
didn't munch the separator paper around the edge. The
cell, such
as it was, started charging at
just 5mA.
Ouch! And I had neglected to measure the internal resistance of
the
monel powder before I had covered it up and got it wet. (Surely
lower
than last time, with all that pressure?) In a while it rose to
9mA.
Load voltages and times very gradually rose.
The
cell, such
as it was, started charging at
just 5mA.
Ouch! And I had neglected to measure the internal resistance of
the
monel powder before I had covered it up and got it wet. (Surely
lower
than last time, with all that pressure?) In a while it rose to
9mA.
Load voltages and times very gradually rose. But
I did make a bracket to hold the 'dremmel' tool on the CNC table's
carriage.
But
I did make a bracket to hold the 'dremmel' tool on the CNC table's
carriage. In the
evening
I decided to drill the holes the immediately fastest
way... by hand again. This time I used a 7/64 inch drill bit.
These
"huge" holes occupying a much greater percentage of the surface
should
let an awful lot more electrolyte through. I glued a bottom piece
on.
In the
evening
I decided to drill the holes the immediately fastest
way... by hand again. This time I used a 7/64 inch drill bit.
These
"huge" holes occupying a much greater percentage of the surface
should
let an awful lot more electrolyte through. I glued a bottom piece
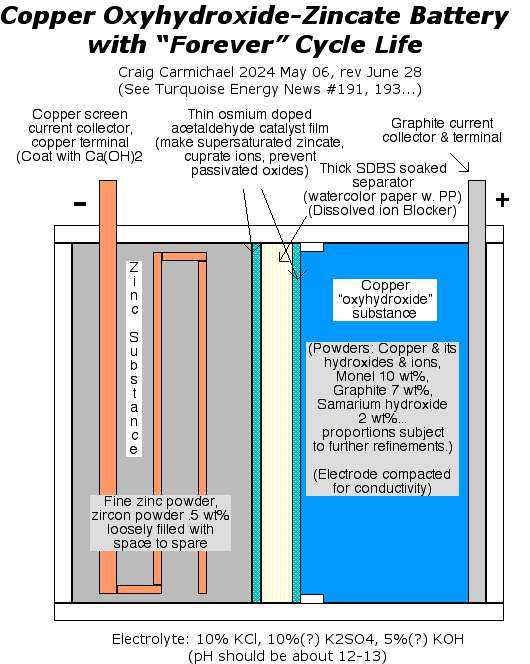
on.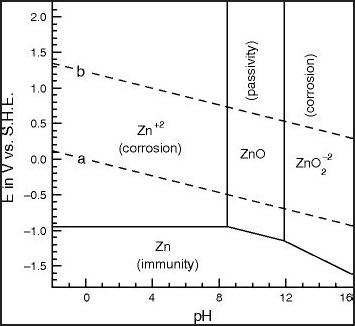 [11th] How
Zinc/Zincate
Electrode Works
[11th] How
Zinc/Zincate
Electrode Works It occurred to me
that if the copper might form Cu(OH)4--, that was pretty
similar to zinc forming Zn(OH)4--. Would it, then, similarly form
a
solution of valence 2 cuprate ions? (or valence 3, perhaps as
Cu(OH)5---?) Would such a solution
gradually turn to "passivated" CuO similar to zinc turning to ZnO,
and
block the electrolyte ions? Dissolved ions wasn't my conception of
what
was happening, but considering that every cell (which now appears
to be
every copper electrode) seems to gradually deteriorate over days,
this
may well be the case.
It occurred to me
that if the copper might form Cu(OH)4--, that was pretty
similar to zinc forming Zn(OH)4--. Would it, then, similarly form
a
solution of valence 2 cuprate ions? (or valence 3, perhaps as
Cu(OH)5---?) Would such a solution
gradually turn to "passivated" CuO similar to zinc turning to ZnO,
and
block the electrolyte ions? Dissolved ions wasn't my conception of
what
was happening, but considering that every cell (which now appears
to be
every copper electrode) seems to gradually deteriorate over days,
this
may well be the case. I
put in three doubled pieces of monel wire, dumped in the 27.5
grams of
powder, and poked it somewhat compact. It only occupied 1/3 of the
tube. I started to conceive that it was going to be something like
the
zinc, dissolving to ions as it charged, plating onto the monel
wires as
it discharged. And so I was probably wrong about the powder
needing to
be compacted much. (Then why was I using the tube shape, again?)
I
put in three doubled pieces of monel wire, dumped in the 27.5
grams of
powder, and poked it somewhat compact. It only occupied 1/3 of the
tube. I started to conceive that it was going to be something like
the
zinc, dissolving to ions as it charged, plating onto the monel
wires as
it discharged. And so I was probably wrong about the powder
needing to
be compacted much. (Then why was I using the tube shape, again?) [14th] I made a
shorter
tube that wouldn't take so darn much monel, and would fit under
the
lid. I didn't put holes near the top. The idea was the electrode
wouldn't come up to the top of the tube. I could put a plug in as
a top
cap, one that would fill the tube with a friction fit, so that
once it
was pushed in it wouldn't willingly push out again and would hold
the
material compacted. If performance dropped it could be pushed
harder,
pushed in a little more, by as much as half an inch.
[14th] I made a
shorter
tube that wouldn't take so darn much monel, and would fit under
the
lid. I didn't put holes near the top. The idea was the electrode
wouldn't come up to the top of the tube. I could put a plug in as
a top
cap, one that would fill the tube with a friction fit, so that
once it
was pushed in it wouldn't willingly push out again and would hold
the
material compacted. If performance dropped it could be pushed
harder,
pushed in a little more, by as much as half an inch. Now
trusting
neither cupro-nickel nor monel - at least not such thin monel wire
- I
thought of the old standby: graphite. I took a 3/8 inch diameter
graphite rod and cut it to 4 inches long, then sanded one end down
to a
point, tapered along the length of the electrode. That way as I
pushed
(or lightly pounded) it in, it would continue to compact the
material a
bit more and at the same time make the best contact. (I had 1/4
&
5/8 inch rod as well, but the pipe was a bigger diameter than I
had
wanted anyway, so a bit of extra filler that also made for a
"thinner"
active electrode and more contact area to the current collector
seemed
to be all to the good.)
Now
trusting
neither cupro-nickel nor monel - at least not such thin monel wire
- I
thought of the old standby: graphite. I took a 3/8 inch diameter
graphite rod and cut it to 4 inches long, then sanded one end down
to a
point, tapered along the length of the electrode. That way as I
pushed
(or lightly pounded) it in, it would continue to compact the
material a
bit more and at the same time make the best contact. (I had 1/4
&
5/8 inch rod as well, but the pipe was a bigger diameter than I
had
wanted anyway, so a bit of extra filler that also made for a
"thinner"
active electrode and more contact area to the current collector
seemed
to be all to the good.) Hmm...
Let's
start at the beginning. I opened up a couple of nickel-metal
hydride
"D" cells and unwrapped the rolled up electrodes in one. I put a
separator paper (toluened, SDBS'ed, PP ironed in, but not
osmiumed)
into the 4 inch PVC tube with the large holes.
Hmm...
Let's
start at the beginning. I opened up a couple of nickel-metal
hydride
"D" cells and unwrapped the rolled up electrodes in one. I put a
separator paper (toluened, SDBS'ed, PP ironed in, but not
osmiumed)
into the 4 inch PVC tube with the large holes. Then I wrapped
some 'sheets' of the nickel hydroxide substance into it around the
outside. I cut a 5 inch section of the 3/8 inch graphite rod,
sanded it
into something of a cone, and put it in the middle. I put in
enough dry
cell stuff that it was a tight fit. The pieces came up to the top
so
rather than make a new end cap I used heat glue to seal the top. I
estimate it was around half the nickel side of one 10 amp-hour "D"
cell, maybe 4 or 5 amp-hours.
Then I wrapped
some 'sheets' of the nickel hydroxide substance into it around the
outside. I cut a 5 inch section of the 3/8 inch graphite rod,
sanded it
into something of a cone, and put it in the middle. I put in
enough dry
cell stuff that it was a tight fit. The pieces came up to the top
so
rather than make a new end cap I used heat glue to seal the top. I
estimate it was around half the nickel side of one 10 amp-hour "D"
cell, maybe 4 or 5 amp-hours. It
didn't seem to work. It was
as if there was no cell connected. I finally wondered if maybe the
nickel side wouldn't work with the SDBS in the separator paper. I
took
it apart and put in an un-SDBS'ed paper. Still nothing. I moved
the
alligator clip connection to the zinc side - again - and suddenly
it
all worked. (Dang alligator clips!) Crap, now I don't know if the
nickel would have a problem with the SDBS. I have no reason to
think it
would, but now it has a paper without it instead of with it. Oh
wait...
the zinc paper has SDBS, so there's an SDBS'ed paper between the
nickel
and zinc anyway. I wonder if a nickel side would last forever if
gelled
with SDBS instead of just for 500-1000 cycles? I don't see why
not.
It
didn't seem to work. It was
as if there was no cell connected. I finally wondered if maybe the
nickel side wouldn't work with the SDBS in the separator paper. I
took
it apart and put in an un-SDBS'ed paper. Still nothing. I moved
the
alligator clip connection to the zinc side - again - and suddenly
it
all worked. (Dang alligator clips!) Crap, now I don't know if the
nickel would have a problem with the SDBS. I have no reason to
think it
would, but now it has a paper without it instead of with it. Oh
wait...
the zinc paper has SDBS, so there's an SDBS'ed paper between the
nickel
and zinc anyway. I wonder if a nickel side would last forever if
gelled
with SDBS instead of just for 500-1000 cycles? I don't see why
not. But what
about
copper? On the fourth I had made a bracket to mount my 'dremmel'
drill
onto the CNC table. Now I decided to make the "rotisserie" for the
CNC
table to drill holes in plastic electrode tubes. I thought of
using a
coupling nut, and after that everything became simple. I only had
3/8"
ones, which turned out to be just right. That was too big for the
stepper motor shaft, but I dug out another stepper with a 3/8"
shaft. I
drilled out the threads so the nut would slip onto the motor
shaft, and
put in a set screw hole & screw to hold it. I would have
rather had
a smaller bolt (1/4") and hence more gap into which to drill the
holes
before hitting it, but the space should be sufficient if all is
carefully set up.
But what
about
copper? On the fourth I had made a bracket to mount my 'dremmel'
drill
onto the CNC table. Now I decided to make the "rotisserie" for the
CNC
table to drill holes in plastic electrode tubes. I thought of
using a
coupling nut, and after that everything became simple. I only had
3/8"
ones, which turned out to be just right. That was too big for the
stepper motor shaft, but I dug out another stepper with a 3/8"
shaft. I
drilled out the threads so the nut would slip onto the motor
shaft, and
put in a set screw hole & screw to hold it. I would have
rather had
a smaller bolt (1/4") and hence more gap into which to drill the
holes
before hitting it, but the space should be sufficient if all is
carefully set up. Then I
found
a piece of 90° angle with holes in it and mounted the motor onto
that. The lower side fits onto bolts on the CNC table. Presto!
Then I
found
a piece of 90° angle with holes in it and mounted the motor onto
that. The lower side fits onto bolts on the CNC table. Presto! [July 1st] I changed
the
program a bit to put a little more space between holes also with a
couple of adjustments to go faster - it had taken over an hour
with
considerable wasted movements. When I went to drill a new tube,
everything went crazy. The drill pulled out of the collet and the
tube
was gouged.
[July 1st] I changed
the
program a bit to put a little more space between holes also with a
couple of adjustments to go faster - it had taken over an hour
with
considerable wasted movements. When I went to drill a new tube,
everything went crazy. The drill pulled out of the collet and the
tube
was gouged. After
that the
real problem turned out to be that while I had edited the G-code
program in the text editor, and told the CNC program to "reload
G-code"
a couple of times, the program had started up by opening a sample
G-code routing program and I hadn't opened the real one. All the
useless and annoying safeties in the CNC program merely distracted
from
the broad view - it did crazy things because it was running the
wrong
G-code file entirely! Why would it load some useless sample
milling
routine as a default instead of starting blank and waiting for the
user
to pick one? Ah well, caveat emptor... It was good to get that
weak
vertical axis fixed anyway as it probably would have messed up
jobs in
the future.
After
that the
real problem turned out to be that while I had edited the G-code
program in the text editor, and told the CNC program to "reload
G-code"
a couple of times, the program had started up by opening a sample
G-code routing program and I hadn't opened the real one. All the
useless and annoying safeties in the CNC program merely distracted
from
the broad view - it did crazy things because it was running the
wrong
G-code file entirely! Why would it load some useless sample
milling
routine as a default instead of starting blank and waiting for the
user
to pick one? Ah well, caveat emptor... It was good to get that
weak
vertical axis fixed anyway as it probably would have messed up
jobs in
the future.

| Days of __ KWH |
June 2024 (18 Collectors) |
May 2024 (18 C's) |
June 2023 (18 C's) |
| 0.xx |
|||
| 1.xx |
|||
| 2.xx |
|||
| 3.xx |
|
||
| 4.xx |
|||
| 5.xx |
|
||
| 6.xx |
|
|
|
| 7.xx |
2 |
1 |
|
| 8.xx |
2 |
1 |
|
| 9.xx |
1 |
3 |
1 |
| 10.xx |
4 |
||
| 11.xx |
1 |
3 |
1 |
| 12.xx |
3 |
1 |
1 |
| 13.xx |
5 |
2 |
2 |
| 14.xx |
3 |
4 |
1 |
| 15.xx |
3 |
2 |
|
| 16.xx |
5 |
1 |
4 |
| 17.xx |
2 |
2 |
2 |
| 18.xx |
1 |
|
2 |
| 19.xx |
1 |
||
| 20.xx |
1 |
1 |
5 |
| 21.xx |
|
1 |
3 |
| 22.xx |
2 |
2 |
|
| 23.xx |
2 |
2 |
|
| 24.xx |
1 |
1 |
|
| 25.xx |
1 |
2 |
|
| 26.xx |
|||
| 27.xx |
|||
| 28.xx |
|||
| 29.xx |
|||
| Total KWH for month |
420 |
405 |
540.12 |
| Km Driven on Electricity |
~1465 Km 190 KWH |
1091.9 ~145 KWH (ODO 108799) |
1407.7 Km (190 KWH?) |